Co-cultivation of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 Causes Differential Gene Expression and Improvement in the Wheat Growth and Biocontrol Activity
- PMID: 31156586
- PMCID: PMC6532653
- DOI: 10.3389/fmicb.2019.01068
Co-cultivation of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 Causes Differential Gene Expression and Improvement in the Wheat Growth and Biocontrol Activity
Abstract
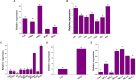
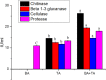
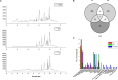
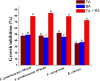
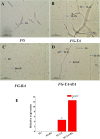
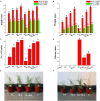
In an effort to balance the demands of plant growth promoting and biological control agents in a single product, the technology on the co-cultivation of two microbes, Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 has been developed and demonstrated its effectiveness in synergistic interactions and its impact on the plant growth and biocontrol potential. In this study, optimization of T. asperellum and B. amyloliquefaciens growth in a single medium was carried out using response surface methodology (RSM). The optimal medium for enhanced growth was estimated as 2% yeast extract, 2% molasses and 2% corn gluten meal. T. asperellum evolved the complicated molecular mechanisms in the co-culture by the induction of BLR-1/BLR-2, VELVET, and NADPH oxidases genes. In performance with these genes, conserved signaling pathways, such as heterotrimeric G proteins and mitogen-activated protein kinases (MAPKs) had also involved in this molecular orchestration. The co-cultivation induced the expression of T. asperellum genes related to secondary metabolism, mycoparasitism, antioxidants and plant growth. On the other hand, the competition during co-cultivation induced the production of new compounds that are not detected in axenic cultures. In addition, the co-culture significantly enhanced the plant growth and protection against Fusarium graminearum. The present study demonstrated the potential of co-cultivation technology could be a used to grow the T. asperellum GDFS1009 and B. amyloliquefaciens 1841 synergistically to improve the production of mycoparasitism related enzymes, secondary metabolites, and plant growth promoting compounds to significantly enhance the plant growth and protection against plant pathogens.
Keywords: B. amyloliquefaciens; T. asperellum; biocontrol; co-cultivation; plant growth.
Figures

References
-
- Baiyee B., Pornsuriya C., Ito S.-I., Sunpapao A. (2019). Trichoderma spirale T76-1 displays biocontrol activity against leaf spot on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol. Control 129 195–200.
LinkOut - more resources
Full Text Sources
Other Literature Sources

