Genome-Wide Characterization and Expression Analysis of Soybean TGA Transcription Factors Identified a Novel TGA Gene Involved in Drought and Salt Tolerance
- PMID: 31156656
- PMCID: PMC6531876
- DOI: 10.3389/fpls.2019.00549
Genome-Wide Characterization and Expression Analysis of Soybean TGA Transcription Factors Identified a Novel TGA Gene Involved in Drought and Salt Tolerance
Abstract
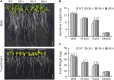
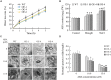
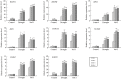
The TGA transcription factors, a subfamily of bZIP group D, play crucial roles in various biological processes, including the regulation of growth and development as well as responses to pathogens and abiotic stress. In this study, 27 TGA genes were identified in the soybean genome. The expression patterns of GmTGA genes showed that several GmTGA genes are differentially expressed under drought and salt stress conditions. Among them, GmTGA17 was strongly induced by both stress, which were verificated by the promoter-GUS fusion assay. GmTGA17 encodes a nuclear-localized protein with transcriptional activation activity. Heterologous and homologous overexpression of GmTGA17 enhanced tolerance to drought and salt stress in both transgeinc Arabidopsis plants and soybean hairy roots. However, RNAi hairy roots silenced for GmTGA17 exhibited an increased sensitivity to drought and salt stress. In response to drought or salt stress, transgenic Arabidopsis plants had an increased chlorophyll and proline contents, a higher ABA content, a decreased MDA content, a reduced water loss rate, and an altered expression of ABA- responsive marker genes compared with WT plants. In addition, transgenic Arabidopsis plants were more sensitive to ABA in stomatal closure. Similarly, measurement of physiological parameters showed an increase in chlorophyll and proline contents, with a decrease in MDA content in soybean seedlings with overexpression hairy roots after drought and salt stress treatments. The opposite results for each measurement were observed in RNAi lines. This study provides new insights for functional analysis of soybean TGA transcription factors in abiotic stress.
Keywords: TGA transcription factor; abiotic stress response; drought and salt tolerance; molecular characterization; soybean.
Figures









Similar articles
-
Expression Analyses of Soybean VOZ Transcription Factors and the Role of GmVOZ1G in Drought and Salt Stress Tolerance.Int J Mol Sci. 2020 Mar 21;21(6):2177. doi: 10.3390/ijms21062177. Int J Mol Sci. 2020. PMID: 32245276 Free PMC article.
-
Genomic Analysis of Stress Associated Proteins in Soybean and the Role of GmSAP16 in Abiotic Stress Responses in Arabidopsis and Soybean.Front Plant Sci. 2019 Nov 18;10:1453. doi: 10.3389/fpls.2019.01453. eCollection 2019. Front Plant Sci. 2019. PMID: 31803204 Free PMC article.
-
Identification and characterization of GmMYB118 responses to drought and salt stress.BMC Plant Biol. 2018 Dec 3;18(1):320. doi: 10.1186/s12870-018-1551-7. BMC Plant Biol. 2018. PMID: 30509166 Free PMC article.
-
Overexpression of the Soybean NAC Gene GmNAC109 Increases Lateral Root Formation and Abiotic Stress Tolerance in Transgenic Arabidopsis Plants.Front Plant Sci. 2019 Aug 16;10:1036. doi: 10.3389/fpls.2019.01036. eCollection 2019. Front Plant Sci. 2019. PMID: 31475026 Free PMC article.
-
Genome-Wide Analysis of the Soybean Calmodulin-Binding Protein 60 Family and Identification of GmCBP60A-1 Responses to Drought and Salt Stresses.Int J Mol Sci. 2021 Dec 16;22(24):13501. doi: 10.3390/ijms222413501. Int J Mol Sci. 2021. PMID: 34948302 Free PMC article.
Cited by
-
Leaf-transcriptome profiles of phoebe bournei provide insights into temporal drought stress responses.Front Plant Sci. 2022 Oct 24;13:1010314. doi: 10.3389/fpls.2022.1010314. eCollection 2022. Front Plant Sci. 2022. PMID: 36352866 Free PMC article.
-
Genome-wide characterization and expression profiling of the TGA gene family in sweet orange (Citrus sinensis) reveal CsTGA7 responses to multiple phytohormones and abiotic stresses.Front Plant Sci. 2025 Feb 25;16:1530242. doi: 10.3389/fpls.2025.1530242. eCollection 2025. Front Plant Sci. 2025. PMID: 40070708 Free PMC article.
-
Hairy Root Transformation: A Useful Tool to Explore Gene Function and Expression in Salix spp. Recalcitrant to Transformation.Front Plant Sci. 2019 Nov 11;10:1427. doi: 10.3389/fpls.2019.01427. eCollection 2019. Front Plant Sci. 2019. PMID: 31781143 Free PMC article.
-
Emerging Roles of Salicylic Acid in Plant Saline Stress Tolerance.Int J Mol Sci. 2023 Feb 8;24(4):3388. doi: 10.3390/ijms24043388. Int J Mol Sci. 2023. PMID: 36834798 Free PMC article. Review.
-
Overexpression of GmUBC9 Gene Enhances Plant Drought Resistance and Affects Flowering Time via Histone H2B Monoubiquitination.Front Plant Sci. 2020 Sep 4;11:555794. doi: 10.3389/fpls.2020.555794. eCollection 2020. Front Plant Sci. 2020. PMID: 33013972 Free PMC article.
References
LinkOut - more resources
Full Text Sources

