The Rhizophagus irregularis Genome Encodes Two CTR Copper Transporters That Mediate Cu Import Into the Cytosol and a CTR-Like Protein Likely Involved in Copper Tolerance
- PMID: 31156674
- PMCID: PMC6531763
- DOI: 10.3389/fpls.2019.00604
The Rhizophagus irregularis Genome Encodes Two CTR Copper Transporters That Mediate Cu Import Into the Cytosol and a CTR-Like Protein Likely Involved in Copper Tolerance
Abstract
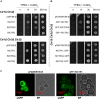
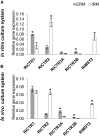
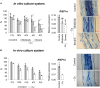
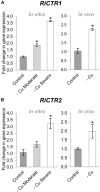
Arbuscular mycorrhizal fungi increase fitness of their host plants under Cu deficient and toxic conditions. In this study, we have characterized two Cu transporters of the CTR family (RiCTR1 and RiCTR2) and a CTR-like protein (RiCTR3A) of Rhizophagus irregularis. Functional analyses in yeast revealed that RiCTR1 encodes a plasma membrane Cu transporter, RiCTR2 a vacuolar Cu transporter and RiCTR3A a plasma membrane protein involved in Cu tolerance. RiCTR1 was more highly expressed in the extraradical mycelia (ERM) and RiCTR2 in the intraradical mycelia (IRM). In the ERM, RiCTR1 expression was up-regulated by Cu deficiency and down-regulated by Cu toxicity. RiCTR2 expression increased only in the ERM grown under severe Cu-deficient conditions. These data suggest that RiCTR1 is involved in Cu uptake by the ERM and RiCTR2 in mobilization of vacuolar Cu stores. Cu deficiency decreased mycorrhizal colonization and arbuscule frequency, but increased RiCTR1 and RiCTR2 expression in the IRM, which suggest that the IRM has a high Cu demand. The two alternatively spliced products of RiCTR3, RiCTR3A and RiCTR3B, were more highly expressed in the ERM. Up-regulation of RiCTR3A by Cu toxicity and the yeast complementation assays suggest that RiCTR3A might function as a Cu receptor involved in Cu tolerance.
Keywords: CTR family; Rhizophagus irregularis; arbuscular mycorrhizal fungi; copper homeostasis; copper transporters; symbiosis.
Figures



References
-
- Balestrini R., Gómez-Ariza J., Lanfranco L., Bonfante P. (2007). Laser microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol. Plant Microb. Interact. 20 1055–1062. 10.1094/mpmi-20-9-1055 - DOI - PubMed

