Effect of intracerebroventricular injection of GABA receptors antagonists on morphine-induced changes in GABA and GLU transmission within the mPFC: an in vivo microdialysis study
- PMID: 31156783
- PMCID: PMC6528724
- DOI: 10.22038/ijbms.2019.28478.6925
Effect of intracerebroventricular injection of GABA receptors antagonists on morphine-induced changes in GABA and GLU transmission within the mPFC: an in vivo microdialysis study
Abstract
Objectives: Many studies have focused on ventral tegmental area than of other mesocorticolimbic areas, and implicated a key role for the medial prefrontal cortex (mPFC) in the development of addictive behaviors. So far, the role of gamma-aminobutyric acid (GABA) receptors in the discriminative properties of morphine has received little attention and few studies evaluated the role of these receptors in drug dependence. Hence, we investigated the role of this receptor on morphine- induced GABA/ glutamate (GLU) changes in the mPFC following morphine administration using in vivo microdialysis.
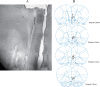
Materials and methods: In this study, 60 rats weighing 270-300 g were divided into six groups. First, microdialysis probe was inserted into the mPFC and was perfused with artificial cerebrospinal fluid and collected the baseline samples in all groups. In saline and morphine groups, the saline, in phaclophen and (phaclofen+morphine) groups, phaclofen (100 nmol), and in bicuculline and (bicuculline+morphine) groups, bicuculline (20 nmol) was injected intracerebroventricular. In saline, phaclofen and bicuculline groups 20 min later, animals received saline (0.2 ml, IP) and others groups received morphine (20 mg/kg, IP).
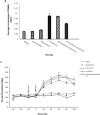
Results: Our results showed that morphine increased the average concentration of GABA and decreased the concentration of GLU within mPFC. Pretreatment with phaclofen and bicuculline 20 min before morphine administration had no effect on GABA and GLU release for 100 min.
Conclusion: The present study indicated that morphine influence the GABA and GLU transmission in mPFC. Therefore evaluation of neurochemistry changes of this neural circuitry may provide further insight into the mechanisms underlying drug dependence.
Keywords: Addiction; GABA-A receptor-antagonists; GABA-B receptor-antagonists; Morphine; Prefrontal cortex.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Gao H, Xiang Y, Sun N, Zhu H, Wang Y, Liu M, et al. Metabolic changes in rat prefrontal cortex and hippocampus induced by chronic morphine treatment studied ex vivo by high resolution 1H NMR spectroscopy. Neurochem Int. 2007;50:386–394. - PubMed
-
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci. Biobehav. Rev. 2010;35:276–284. - PubMed
-
- Fattore L, Fadda P, Spano M, Pistis M, Fratta W. Neurobiological mechanisms of cannabinoid addiction. Mol Cell Endocrinol. 2008;286:97–107. - PubMed
LinkOut - more resources
Full Text Sources
