Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids
- PMID: 31156789
- PMCID: PMC6528723
- DOI: 10.22038/ijbms.2019.32873.7852
Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids
Abstract
Objectives: Curcuminoids, comprising curcumin, demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC), are bioactive phytochemicals with numerous pharmacological effects. Oral biological availability of curcuminoids is low due to the low aqueous solubility and rapid metabolism. This study aimed at fabricating a nanomicellar curcuminoid formula with enhanced pharmacokinetic properties.
Materials and methods: Curcuminoids nanomicelles were prepared and characterized regarding particle properties, stability, release profile and pharmacokinetic parameters.
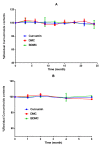
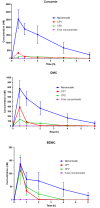
Results: Encapsulation efficiency of curcuminoids in nanomicelles were 100%. Particle size analysis demonstrated a mean size of around 10 nm that remained stable for 24 months. Dissolution test showed the complete dissolution of encapsulated curcuminoids from nanomicelles within 20 min while the free curcuminoids were poorly dissolved (approximately 7% after 60 min). The results of long-term (24 months) and accelerated (6 months) stability studies showed no changes in the size and content of nanomicelles. The release studies in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) showed no release of curcuminoids for at least 4 hours. In vivo study in BALB/c mice showed improved pharmacokinetic parameters including maximum plasma concentration (Cmax) and time to reach the maximum concentration (Tmax) with nanomicelles as compared to free curcuminoids and two other commercial products. Tmax for all the three curcuminoid components was observed 30 min following oral administration. AUC of nanomicellar curcuminoids was 59.2 times more than free curcuminoids.
Conclusion: These data indicated that nanomicelles could improve solubility, oral bioavailability and also the stability of curcuminoids. Thus, they merit further investigation for enhancing pharmacological effects of curcuminoids.
Keywords: Biological availability; Curcuminoid; Drug stability; Micelle; Pharmacokinetics.
Conflict of interest statement
The authors report no declarations of interest.
Figures




References
-
- Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2–13. - PubMed
-
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–1064. - PubMed
-
- Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother Res. 2014;28:633–642. - PubMed
-
- Panahi Y, Sahebkar A, Parvin S, Saadat A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann Clin Biochem . 2012;49:580–588. - PubMed
LinkOut - more resources
Full Text Sources
