Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia
- PMID: 31156798
- PMCID: PMC6515841
- DOI: 10.1177/2040620719837906
Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia
Abstract
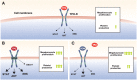
Primary immune thrombocytopenia (ITP) is a bleeding disorder that conventionally has been treated with steroids or other immunosuppressive treatments. The introduction of thrombopoietin receptor agonists (TPO-RAs), which increase platelet production, dramatically changed the treatment landscape for ITP by providing patients with well-tolerated, long-term treatment options. Two TPO-RAs, eltrombopag and romiplostim, have been approved in the United States and European Union for the treatment of ITP. Some patients do not benefit from the first TPO-RA they receive, so it is assumed that the alternate TPO-RA would have the same outcome. However, eltrombopag and romiplostim have distinct pharmacodynamic and pharmacokinetic properties and may have different tolerability and efficacy in individual patients with ITP. Published retrospective studies showed that >75% of patients who switched to the alternate TPO-RA maintained or achieved a response with the new treatment. Notably, most patients who switched due to lack of efficacy with the first TPO-RA responded to the alternate TPO-RA, which demonstrates an absence of cross-resistance between the two drugs. Therefore, switching to the alternate TPO-RA if the first TPO-RA fails to demonstrate a response should be considered before the use of a less-preferable option.
Keywords: eltrombopag; hemorrhage; immune thrombocytopenia; romiplostim; thrombopoietin.
Conflict of interest statement
Conflict of interest statement: Dr. González-Porras and Dr. Carpenedo have nothing to disclose. Dr. Godeau reports grants and personal fees from Amgen, personal fees from Novartis, personal fees from LFB, grants and personal fees from Roche, and personal fees from Argenx, outside the submitted work.
Figures

References
-
- Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol 2013; 98: 10–23. - PubMed
-
- Nichol JL. Endogenous TPO (eTPO) levels in health and disease: possible clues for therapeutic intervention. Stem Cells Dayt Ohio 1998; 16(Suppl. 2): 165–175. - PubMed
-
- Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011; 117: 4190–4207. - PubMed
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009; 113: 2386–2393. - PubMed
-
- Rodeghiero F, Ruggeri M. Treatment of immune thrombocytopenia in adults: the role of thrombopoietin-receptor agonists. Semin Hematol 2015; 52: 16–24. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

