A retrospective and observational analysis of harmful excipients in medicines for hospitalised neonates in Latvia
- PMID: 31157015
- PMCID: PMC6452392
- DOI: 10.1136/ejhpharm-2016-001107
A retrospective and observational analysis of harmful excipients in medicines for hospitalised neonates in Latvia
Abstract
Background: Medicines used in neonates contain different excipients, which may not be safe in this age group.
Objective: To analyse the frequency at which hospitalised neonates are exposed to harmful excipients (HEs) and to identify substitution possibilities for medicines containing HEs.
Materials and methods: Retrospective, observational study at a university paediatric hospital from 1 September 2015 till 29 February 2016. All hospitalised neonates who received a prescription for medicines containing an HE were included. Neonates were divided into four groups according to gestational age (<28 weeks; 28 to <32 weeks; 32 to <37 weeks and ≥37 weeks). The following excipients were analysed: parabens, polysorbate 80, propylene glycol, benzoates, saccharin sodium, sorbitol, ethanol and benzalkonium chloride. Excipients were identified from the Summaries of Product Characteristics.
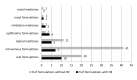
Results: 296 (102(34.5%) preterm) neonates included in the study received 1472 prescriptions for 106 medicines. The most often used formulations were intravenous (48/106; 45.3%) and oral solid formulations (20; 18.9%). The total number of different excipients was 169. In total, 29/106 (27.4%) medicines contained at least one HE. In total 82/102 (80.4%) preterm and 118/194 (60.8%) term neonates received medications with at least one HE. Substitution was possible for 9/29 (31.0%) HE-containing medicines.
Conclusions: Use of HEs can be reduced by using HE-free products available on the European market. However, medicine substitution was possible in only a small number of cases. Therefore the main focus should be on information and education of the hospital specialists about HEs used in medicines and their adverse reactions.
Keywords: harmful excipients; hospitals; neonates; pharmaceutical excipients; substitution of harmful excipients.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Alade SL, Brown RE, Paquet A Jr. Polysorbate 80 and E-Ferol toxicity. Pediatrics 1986;77:593–7. - PubMed
LinkOut - more resources
Full Text Sources