Analysis of self-reported adverse reactions to efavirenz and drug interactions in a population with HIV in Mexico
- PMID: 31157050
- PMCID: PMC6319403
- DOI: 10.1136/ejhpharm-2016-001106
Analysis of self-reported adverse reactions to efavirenz and drug interactions in a population with HIV in Mexico
Abstract
Objective: To analyse the most frequent self-reported adverse reactions (ARs), the durability and the causes of antiretrovirals (ARVs) regimens change, concomitant treatments and drug interactions related to the use of ARVs in a group of people living with HIV in Cuernavaca, Morelos, Mexico.
Materials and methods: Cross-sectional study conducted in a clinic specialising in HIV 'CAPASITS-Cuernavaca' in Mexico from February to June 2015. People who wanted to participate were given a questionnaire on demographic characteristics, adherence, concomitant treatments and ARs. To understand the clinical variables, the clinical records were reviewed. Quantitative variables were compared using Student's t-test for normal data and the Mann-Whitney U test for non-normal data. For comparisons between categorical variables, the χ2 test was used. All tests used a significance level of 0.05.
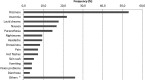
Results: A total of 96 people participated, and 218 ARs (mean= 2.3±1.9) were found. The most frequently encountered ARs were dizziness (53.1%), insomnia (21.9%) and lucid dreams (17.7%). Twenty-three people (24%) were polymedicated, and 18 potential interactions were detected in 12 people.
Conclusions: The results suggest that a thorough analysis of the possible drug interactions should be performed for polymedicated people on ARV treatment and that a protocol should be designed for the monitoring and management of AR to ensure a good adherence to ARV treatment.
Keywords: HIV; adverse reactions; antiretroviral; drug interactions; polymedication.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Guía de manejo antirretroviral de las personas con VIH. México: Censida/Secretaría de Salud, 2014. www.censida.salud.gob.mx/descargas/principal/Guia_ARV_2014V8.pdf (accesed sep 10 2016).
LinkOut - more resources
Full Text Sources
Research Materials