Investigations into the physical and chemical stability of concentrated co-trimoxazole intravenous infusions
- PMID: 31157078
- PMCID: PMC6319416
- DOI: 10.1136/ejhpharm-2017-001225
Investigations into the physical and chemical stability of concentrated co-trimoxazole intravenous infusions
Abstract
Objectives: High dose of intravenous sulfamethoxazole and trimethoprim (co-trimoxazole) is often used in immunocompromised patients for the treatment of Pneumocystis jiroveci pneumonia. Current manufacturer's dilution recommendation for intravenous co-trimoxazole (1:25 v/v) requires the administration of 2 L of additional fluid per day causing serious complications including pulmonary oedema. Intravenous administration of concentrated solution of co-trimoxazole may minimise the risk of fluid overload associated side effects. Therefore, the objective of the study was to investigate the physicochemical stability of concentrated intravenous co-trimoxazole solutions.
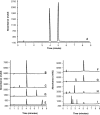
Methods: Four ampoules of intravenous co-trimoxazole were injected into an infusion bag containing either 480 (1:25 v/v), 380 (1:20 v/v), 280 (1:15 v/v) or 180 (1:10 v/v) mL of glucose 5% solution. Three bags for each dilution (total 12 bags) were prepared and stored at room temperature. An aliquot was withdrawn immediately (at 0 hour) and after 0.5, 1, 2 and 4 hours of storage for high-performance liquid-chromatography (HPLC) analysis, and additional samples were withdrawn every half an hour for microscopic examination. Each sample was analysed for the concentration of trimethoprim and sulfamethoxazole using a stability indicating HPLC method. Samples were assessed for pH, change in colour (visually) and for particle content (microscopically) immediately after preparation and on each time of analysis.
Results: Intravenous co-trimoxazole at 1:25, 1:20, 1:15 and 1:10 v/v retained more than 98% of the initial concentration of trimethoprim and sulfamethoxazole for 4 hours. There was no major change in pH at time zero and at various time points. Microscopically, no particles were detected for at least 4 hours and 2 hours when intravenous co-trimoxazole was diluted at 1:25 or 1:20 and 1:15 v/v, respectively. More than 1200 particles/mL were detected after 2.5 hours of storage when intravenous co-trimoxazole was diluted at 1:15 v/v.
Conclusions: Intravenous co-trimoxazole is stable over a period of 4 hours when diluted with 380 mL of glucose 5% solution (1:20 v/v) and for 2 hours when diluted with 280 mL glucose 5% solution (1:15 v/v).
Keywords: High Performance Liquid Chromatography; Pneumocystis Jiroveci Pneumonia; Stability And Incompatibility; Sulfamethoxazole; Trimethoprim.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. 2016;Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2016. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf (accessed 3 Nov 2016).
LinkOut - more resources
Full Text Sources
