Validation of a procedure to mix homogenous solutions in bags and syringes
- PMID: 31157082
- PMCID: PMC6319419
- DOI: 10.1136/ejhpharm-2017-001284
Validation of a procedure to mix homogenous solutions in bags and syringes
Abstract
Objectives: It is essential to obtain homogeneous drug mixtures, especially when only a fraction of the prepared dose is to be administered. This study aimed to validate a manual mixing method for guaranteeing homogeneity.
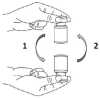
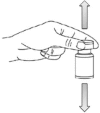
Methods: One operator tested six standardised manual mixing techniques (one, five and 10 inversions, and one, five and 10 bottoms-up agitations) six times each for preparations in bags and syringes. The mixing step was reproduced experimentally by adding a small volume of analyte (0.8 mL for syringes and 6 mL for bags) to a large volume of matrix (50 mL for syringes and 300 mL for bags). Three analyte/matrix pairings were tested: water/water, water/glucose 20% and glucose 20%/water. The tracer (sodium chloride) was assayed using capillary electrophoresis. Volume measurement errors were corrected by weighing bags and syringes. In order to evaluate inter-individual variability, the 10 inversions technique was tested by 10 drug preparation technicians. Mixtures were considered acceptable if they were between 95% and 105% accurate and if the coefficient of variation was ≤5% of the average of the six repetitions.
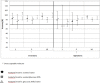
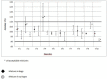
Results: Both the 10 inversions and 10 bottoms-up agitations mixing techniques ensured acceptable mixtures by the principal technician in all tested conditions. When mixing using the 10 inversions method was tested by the 10 technicians, the mixture's mean acceptability could no longer be ensured.
Conclusion: Use of a standardised mixing technique did not appear to be sufficient to obtain a homogeneous mixture across technicians. Standardised guidelines for needle position, needle rinsing and speed of addition should be implemented.
Keywords: bags; drugs; mixtures; standardized manual mixing technique; syringes.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- WHO Expert Committee on specifications for Pharmaceutical Preparations. WHO good manufacturing practices for pharmaceutical products: main principles, 2011.
-
- Bergman N, Vellar ID. Potential life-threatening variations of drug concentrations in intravenous infusion systems: potassium chloride, insulin, and heparin. Med J Aust 1982;2:270–2. - PubMed
-
- Deardorff DL, Schmidt CN, Wiley RA. Effect of preparation techniques on mixing of additives in intravenous fluids in nonrigid containers. Hosp Pharm 1993;28:309–10. - PubMed
LinkOut - more resources
Full Text Sources
