Telomerase and Telomeres in Endometrial Cancer
- PMID: 31157162
- PMCID: PMC6533802
- DOI: 10.3389/fonc.2019.00344
Telomerase and Telomeres in Endometrial Cancer
Abstract
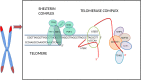
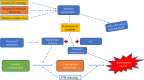
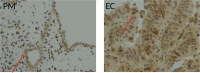
Telomeres at the termini of human chromosomes are shortened with each round of cell division due to the "end replication problem" as well as oxidative stress. During carcinogenesis, cells acquire or retain mechanisms to maintain telomeres to avoid initiation of cellular senescence or apoptosis and halting cell division by critically short telomeres. The unique reverse transcriptase enzyme complex, telomerase, catalyzes the maintenance of telomeres but most human somatic cells do not have sufficient telomerase activity to prevent telomere shortening. Tissues with high and prolonged replicative potential demonstrate adequate cellular telomerase activity to prevent telomere erosion, and high telomerase activity appears to be a critical feature of most (80-90%) epithelial cancers, including endometrial cancer. Endometrial cancers regress in response to progesterone which is frequently used to treat advanced endometrial cancer. Endometrial telomerase is inhibited by progestogens and deciphering telomere and telomerase biology in endometrial cancer is therefore important, as targeting telomerase (a downstream target of progestogens) in endometrial cancer may provide novel and more effective therapeutic avenues. This review aims to examine the available evidence for the role and importance of telomere and telomerase biology in endometrial cancer.
Keywords: TERRA; TRAP; endometrial cancer; endometrium; hTERC; hTERT; telomerase; telomere.
Figures
Similar articles
-
Regulation and Effect of Telomerase and Telomeric Length in Stem Cells.Curr Stem Cell Res Ther. 2021;16(7):809-823. doi: 10.2174/1574888X15666200422104423. Curr Stem Cell Res Ther. 2021. PMID: 32321410 Review.
-
Implications of telomeres and telomerase in endometrial pathology.Hum Reprod Update. 2017 Mar 1;23(2):166-187. doi: 10.1093/humupd/dmw044. Hum Reprod Update. 2017. PMID: 27979878 Free PMC article. Review.
-
Tissue formation and tissue engineering through host cell recruitment or a potential injectable cell-based biocomposite with replicative potential: Molecular mechanisms controlling cellular senescence and the involvement of controlled transient telomerase activation therapies.J Biomed Mater Res A. 2015 Dec;103(12):3993-4023. doi: 10.1002/jbm.a.35515. Epub 2015 Aug 14. J Biomed Mater Res A. 2015. PMID: 26034007 Review.
-
The role of telomeres and telomerase complex in haematological neoplasia: the length of telomeres as a marker of carcinogenesis and prognosis of disease.Prague Med Rep. 2010;111(2):91-105. Prague Med Rep. 2010. PMID: 20653999 Review.
-
Role of Telomeres and Telomeric Proteins in Human Malignancies and Their Therapeutic Potential.Cancers (Basel). 2020 Jul 14;12(7):1901. doi: 10.3390/cancers12071901. Cancers (Basel). 2020. PMID: 32674474 Free PMC article. Review.
Cited by
-
Human Uterine Biopsy: Research Value and Common Pitfalls.Int J Reprod Med. 2020 Apr 28;2020:9275360. doi: 10.1155/2020/9275360. eCollection 2020. Int J Reprod Med. 2020. PMID: 32411783 Free PMC article. Review.
-
Telomeres, telomerase, and cancer: mechanisms, biomarkers, and therapeutics.Exp Hematol Oncol. 2025 Jan 27;14(1):8. doi: 10.1186/s40164-025-00597-9. Exp Hematol Oncol. 2025. PMID: 39871386 Free PMC article. Review.
-
Identification of novel key genes associated with uterine corpus endometrial carcinoma progression and prognosis.Ann Transl Med. 2023 Jan 31;11(2):100. doi: 10.21037/atm-22-6461. Ann Transl Med. 2023. PMID: 36819577 Free PMC article.
-
Test Strip Platform Spin-Off for Telomerase Activity Detection: Development of an Electrochemical Biosensor.ACS Omega. 2022 Mar 9;7(11):9964-9972. doi: 10.1021/acsomega.2c00713. eCollection 2022 Mar 22. ACS Omega. 2022. PMID: 35356692 Free PMC article.
-
Telomere and Telomerase-Associated Proteins in Endometrial Carcinogenesis and Cancer-Associated Survival.Int J Mol Sci. 2022 Jan 6;23(2):626. doi: 10.3390/ijms23020626. Int J Mol Sci. 2022. PMID: 35054812 Free PMC article.
References
-
- van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. (1998) 92:401–13. - PubMed
-
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. (1999) 283:1321–5. - PubMed
-
- Sandell LL, Zakian V A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. (1993) 75:729–39. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

