The Oncogene IARS2 Promotes Non-small Cell Lung Cancer Tumorigenesis by Activating the AKT/MTOR Pathway
- PMID: 31157169
- PMCID: PMC6528107
- DOI: 10.3389/fonc.2019.00393
The Oncogene IARS2 Promotes Non-small Cell Lung Cancer Tumorigenesis by Activating the AKT/MTOR Pathway
Abstract
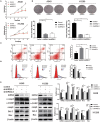
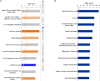
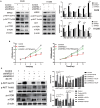
A limited number of studies have indicated an association between isoleucyl-tRNA synthetase 2 (IARS2) and tumorigenesis. We evaluated IARS2 protein expression in lung tumor tissues and paired non-tumor tissues. We found higher IARS2 expression in the tumor tissues, which was associated with the late Tumor and Node stages of the Tumor, Node, Metastasis staging system. Silencing IARS2 inhibited the activity of A549 and H1299 cells, resulting in G0/G1 stasis of A549 cells and mitochondrial apoptosis. IARS2 silencing was also found to inhibit NSCLC tumor growth in nude mice. Complementary DNA microarray analysis revealed 742 differentially expressed genes (507 upregulated and 235 downregulated) in IARS2-silenced A549 cells compared to controls. Ingenuity Pathway Analysis of the differential expression data suggested that multiple pathways are associated with IARS2 silencing in NSCLC cells; upstream analysis predicted the activation or inhibition of transcriptional regulators. Correlation analysis revealed that AKT and MTOR activities were significantly inhibited in IARS2-silenced cells, but were partially restored by the AKT-stimulating agent SC79. IARS2 appears to regulate lung cancer cell proliferation via the AKT/MTOR pathway. Our results help clarify the complex roles of IARS2 in tumorigenesis and suggest that it may be a novel regulator of lung cancer development.
Keywords: AKT; cDNA microarray; ingenuity pathway analysis; isoleucyl-tRNA synthetase 2; lung cancer; mammalian target of rapamycin; tumorigenesis.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

