Radiological and Thermal Dose Correlations in Pallidothalamic Tractotomy With MRgFUS
- PMID: 31157233
- PMCID: PMC6533852
- DOI: 10.3389/fsurg.2019.00028
Radiological and Thermal Dose Correlations in Pallidothalamic Tractotomy With MRgFUS
Abstract
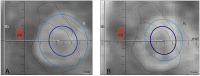
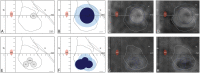
Background: MR-guided focused ultrasound (MRgFUS) offers the possibility of safe and accurate lesioning inside the brain. Until now, most MRgFUS thermal applications have been based on temperature or energy protocols. Experimental studies support however an approach centered on thermal dose control. Objective: To show the technical feasibility and lesion size predictability of a thermal dose approach during MRgFUS pallidothalamic tractotomy (PTT) against chronic therapy-resistant Parkinson's disease (PD). Methods: MR and thermal dose data were analyzed in 31 MRgFUS interventions between January and December 2017 in patients suffering from chronic therapy-resistant Parkinson's disease (PD) using a standardized PTT target covered by 5 to 7 target lesion sub-units. Results: Good correlations were found between (1) the mean axial T2 lesion diameter intraoperatively and the mean 240 cumulative equivalent min at 43°C (240 CEM) thermal dose diameter (r = 0.52), (2) the mean axial T2 diameter 48 h post-treatment and the mean 18 CEM thermal dose diameter (r = 0.62), and (3) the mean axial T2 diameter intraoperatively and 48 h post-treatment (r = 0.62). Conclusion: Our current approach using a thermal dose steering for multiple target lesion sub-units could be reproduced in 31 interventions with a good lesion size predictability.
Keywords: MRgFUS (magnetic resonance-guided focused ultrasound surgery); Parkinson's disease; functional neurosurgery; imaging; incisionless; pallidothalamic tractotomy; radiology; thermal dose.
Figures




References
-
- Meshorer A, Prionas SD, Fajardo LF, Meyer JL, Hahn GM, Martinez AA. The effects of hyperthermia on normal mesenchymal tissues. Application of a histologic grading system. Arch Pathol Lab Med. (1983) 107:328–34. - PubMed
LinkOut - more resources
Full Text Sources

