A paradigm shift in biomarker guided oncology drug development
- PMID: 31157269
- PMCID: PMC6511563
- DOI: 10.21037/atm.2019.03.36
A paradigm shift in biomarker guided oncology drug development
Abstract
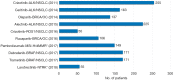
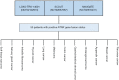
Over the past couple of decades, biomarker driven enrichment clinical trials have proven to be an important tool in clinical drug development, especially for targeted anti-cancer drugs. By the end of 2018, more than 30 drugs have been developed in conjunction with a biomarker test and have a regulatory approved companion diagnostic linked to their use. With the recent approval of larotrectinib (Vitrakvi, Loxo Oncology/Bayer) for patients with neurotrophic receptor tyrosine kinase (NTRK) gene fusion and pembrolizumab (Keytruda, MSD) for microsatellite instability-high (MSI-H) and mis-match-repair-deficient (dMMR) positive patients, we are experiencing a paradigm shift in biomarker guided drug development. In contrast to the previous drugs, they are not developed for a conventional cancer indication defined by tumor histology and anatomical location, but solely on their effect related to specific molecular aberrations. For larotrectinib efficacy was demonstrated across 12 different conventional cancer indications and for pembrolizumab the number was 15. Due to the low prevalence of the different molecular aberrations, data from several small "basket" trials was pooled in order to document the efficacy of the two drugs. With the approval of larotrectinib and the MSI-H/dMMR indication for pembrolizumab, the translational research methodology has demonstrated its potential in relation to drug development and made the way for a more precise and individualized anti-cancer therapy.
Keywords: Larotrectinib; companion diagnostics; microsatellite instability-high (MSI-H); mis-match-repair-deficient (dMMR); neurotrophic receptor tyrosine kinase (NTRK); pembrolizumab; personalized medicine.
Conflict of interest statement
Conflicts of Interest: Jan Trøst Jørgensen has worked as a consultant for Agilent Technologies, Euro Diagnostica, Azanta and Oncology Venture and has given lectures at meetings sponsored by AstraZeneca, Merck Sharp & Dohme, and Roche.
Figures

References
-
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US); 2016. FDA-NIH Biomarker Working Group. 2016 Jan 28. Updated 2017 Sep 25. Available online: https://www.ncbi.nlm.nih.gov/books/NBK338449/. Accessed February 21, 2019. - PubMed
-
- Jørgensen JT, Winther H. The development of the HercepTest – from bench to bedside. In: Jørgensen JT, Winther H. editors. Molecular Diagnostics: The Key Driver in Personalized Cancer Medicine. Pan Stanford, 2010:43-60.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
