Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study
- PMID: 31157583
- PMCID: PMC6774816
- DOI: 10.1200/JCO.19.00882
Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study
Abstract
Purpose: The KRISTINE study compared neoadjuvant trastuzumab emtansine plus pertuzumab (T-DM1+P) with docetaxel, carboplatin, trastuzumab plus P (TCH+P) for the treatment human epidermal growth factor receptor 2-positive stage II to III breast cancer. T-DM1+P led to a lower pathologic complete response rate (44.4% v 55.7%; P = .016), but fewer grade 3 or greater and serious adverse events (AEs). Here, we present 3-year outcomes from KRISTINE.
Methods: Patients were randomly assigned to neoadjuvant T-DM1+P or TCH+P every 3 weeks for six cycles. Patients who received T-DM1+P continued adjuvant T-DM1+P, and patients who received TCH+P received adjuvant trastuzumab plus pertuzumab. Secondary end points included event-free survival (EFS), overall survival, patient-reported outcomes (measured from random assignment), and invasive disease-free survival (IDFS; measured from surgery).
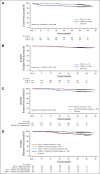
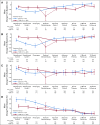
Results: Of patients, 444 were randomly assigned (T-DM1+P, n = 223; TCH+P, n = 221). Median follow-up was 37 months. Risk of an EFS event was higher with TDM-1+P (hazard ratio [HR], 2.61 [95% CI, 1.36 to 4.98]) with more locoregional progression events before surgery (15 [6.7%] v 0). Risk of an IDFS event after surgery was similar between arms (HR, 1.11 [95% CI, 0.52 to 2.40]). Pathologic complete response was associated with a reduced risk of an IDFS event (HR, 0.24 [95% CI, 0.09 to 0.60]) regardless of treatment arm. Overall, grade 3 or greater AEs (31.8% v 67.7%) were less common with T-DM1+P. During adjuvant treatment, grade 3 or greater AEs (24.5% v 9.9%) and AEs leading to treatment discontinuation (18.4% v 3.8%) were more common with T-DM1+P. Patient-reported outcomes favored T-DM1+P during neoadjuvant treatment and were similar to trastuzumab plus pertuzumab during adjuvant treatment.
Conclusion: Compared with TCH+P, T-DM1+P resulted in a higher risk of an EFS event owing to locoregional progression events before surgery, a similar risk of an IDFS event, fewer grade 3 or greater AEs during neoadjuvant treatment, and more AEs leading to treatment discontinuation during adjuvant treatment.
Trial registration: ClinicalTrials.gov NCT02131064.
Figures
Comment in
-
Implications of Neoadjuvant Therapy in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer.J Clin Oncol. 2019 Sep 1;37(25):2189-2192. doi: 10.1200/JCO.19.01159. Epub 2019 Jun 3. J Clin Oncol. 2019. PMID: 31157582 No abstract available.
References
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Breast cancer (version 4.2018) https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf - PubMed
-
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. - PubMed
-
- Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: Results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. - PubMed
-
- Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: Results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. - PubMed
-
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

