GRAZAX®: a sublingual immunotherapy vaccine for Hay fever treatment: from concept to commercialization
- PMID: 31157592
- PMCID: PMC6930101
- DOI: 10.1080/21645515.2019.1622976
GRAZAX®: a sublingual immunotherapy vaccine for Hay fever treatment: from concept to commercialization
Abstract
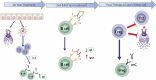
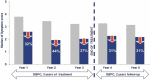
Allergen immunotherapy has been used for more than 100 y, but only recently underlying immunological mechanisms have started to be understood. New Allergy vaccines are now considered to be full pharmaceutical products, that should comply with general as well as specific pharmaceutical legal framework. GRAZAX® is the first global allergy vaccine developed in compliance with the new legal environment and is thus a reference for developing new allergy vaccines. Here, we provide a rationale description of GRAZAX®, providing a sequential description of its pharmaceutical and clinical development. With more than 25 clinical trials, involving more than 8000 patients, including as well three 5-y prospective clinical trials, GRAZAX® is a key product to understand the unique position of allergen-specific immunotherapy as a disease-modifying intervention.
Keywords: Grazax; allergen immunotherapy; allergy vaccine; grass allergy; sublingual immunotherapy.
Figures


References
-
- Bonertz A, Roberts GC, Hoefnagel M, Timon M, Slater JE, Rabin RL, Bridgewater J, Pini C, Pfaar O, Akdis C, Goldstein J.. Challenges in the implementation of EAACI guidelines on allergen immunotherapy: a global perspective on the regulation of allergen products. Allergy. 2018;73:64–76. doi: 10.1111/all.13266. - DOI - PubMed
-
- Bonertz A, Roberts G, Slater JE, Bridgewater J, Rabin RL, Hoefnagel M, Timon M, Pini C, Pfaar O, Sheikh A, Ryan D. Allergen manufacturing and quality aspects for allergen immunotherapy in Europe and the United States: an analysis from the EAACI AIT guidelines project. Allergy. 2018;73:816–26. doi: 10.1111/all.13357. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
