Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer
- PMID: 31157963
- PMCID: PMC6810605
- DOI: 10.1056/NEJMoa1903387
Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer
Abstract
Background: Patients with a germline BRCA1 or BRCA2 mutation make up a small subgroup of those with metastatic pancreatic cancer. The poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitor olaparib has had antitumor activity in this population.
Methods: We conducted a randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy of olaparib as maintenance therapy in patients who had a germline BRCA1 or BRCA2 mutation and metastatic pancreatic cancer and disease that had not progressed during first-line platinum-based chemotherapy. Patients were randomly assigned, in a 3:2 ratio, to receive maintenance olaparib tablets (300 mg twice daily) or placebo. The primary end point was progression-free survival, which was assessed by blinded independent central review.
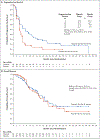
Results: Of the 3315 patients who underwent screening, 154 underwent randomization and were assigned to a trial intervention (92 to receive olaparib and 62 to receive placebo). The median progression-free survival was significantly longer in the olaparib group than in the placebo group (7.4 months vs. 3.8 months; hazard ratio for disease progression or death, 0.53; 95% confidence interval [CI], 0.35 to 0.82; P = 0.004). An interim analysis of overall survival, at a data maturity of 46%, showed no difference between the olaparib and placebo groups (median, 18.9 months vs. 18.1 months; hazard ratio for death, 0.91; 95% CI, 0.56 to 1.46; P = 0.68). There was no significant between-group difference in health-related quality of life, as indicated by the overall change from baseline in the global quality-of-life score (on a 100-point scale, with higher scores indicating better quality of life) based on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (between-group difference, -2.47 points; 95% CI, -7.27 to 2.33). The incidence of grade 3 or higher adverse events was 40% in the olaparib group and 23% in the placebo group (between-group difference, 16 percentage points; 95% CI, -0.02 to 31); 5% and 2% of the patients, respectively, discontinued the trial intervention because of an adverse event.
Conclusions: Among patients with a germline BRCA mutation and metastatic pancreatic cancer, progression-free survival was longer with maintenance olaparib than with placebo. (Funded by AstraZeneca and others; POLO ClinicalTrials.gov number, NCT02184195.).
Copyright © 2019 Massachusetts Medical Society.
Figures

Comment in
-
Maintenance Olaparib New Standard in Pancreatic Cancer?Cancer Discov. 2019 Aug;9(8):OF6. doi: 10.1158/2159-8290.CD-NB2019-065. Epub 2019 Jun 4. Cancer Discov. 2019. PMID: 31164341
-
PARP inhibition - opportunities in pancreatic cancer.Nat Rev Clin Oncol. 2019 Oct;16(10):595-596. doi: 10.1038/s41571-019-0257-6. Nat Rev Clin Oncol. 2019. PMID: 31332344 No abstract available.
-
Maintenance Olaparib for Metastatic Pancreatic Cancer.N Engl J Med. 2019 Oct 10;381(15):1491. doi: 10.1056/NEJMc1911185. N Engl J Med. 2019. PMID: 31597027 No abstract available.
-
Maintenance Olaparib for Metastatic Pancreatic Cancer.N Engl J Med. 2019 Oct 10;381(15):1491-1492. doi: 10.1056/NEJMc1911185. N Engl J Med. 2019. PMID: 31597028 No abstract available.
-
Pancreatic cancer patients with germline BRCA mutations can benefit from olaparib treatment.Transl Cancer Res. 2020 Apr;9(4):2154-2156. doi: 10.21037/tcr.2020.03.07. Transl Cancer Res. 2020. PMID: 35117572 Free PMC article. No abstract available.
-
Bevacizumab as adjunct to chemotherapy for chemoresistant ovarian cancer.Transl Cancer Res. 2020 Apr;9(4):2157-2160. doi: 10.21037/tcr.2020.02.75. Transl Cancer Res. 2020. PMID: 35117573 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
