Emergent decision-making behaviour and rhythm generation in a computational model of the ventromedial nucleus of the hypothalamus
- PMID: 31158265
- PMCID: PMC6564049
- DOI: 10.1371/journal.pcbi.1007092
Emergent decision-making behaviour and rhythm generation in a computational model of the ventromedial nucleus of the hypothalamus
Abstract
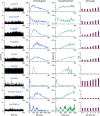
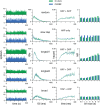
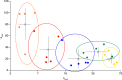
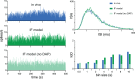
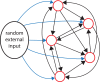
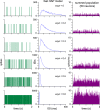
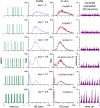
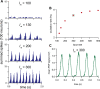
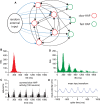
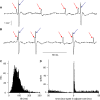
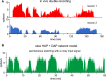
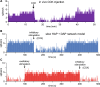
The ventromedial nucleus of the hypothalamus (VMN) has an important role in diverse behaviours. The common involvement in these of sex steroids, nutritionally-related signals, and emotional inputs from other brain areas, suggests that, at any given time, its output is in one of a discrete number of possible states corresponding to discrete motivational drives. Here we explored how networks of VMN neurons might generate such a decision-making architecture. We began with minimalist assumptions about the intrinsic properties of VMN neurons inferred from electrophysiological recordings of these neurons in rats in vivo, using an integrate-and-fire based model modified to simulate activity-dependent post-spike changes in neuronal excitability. We used a genetic algorithm based method to fit model parameters to the statistical features of spike patterning in each cell. The spike patterns in both recorded cells and model cells were assessed by analysis of interspike interval distributions and of the index of dispersion of firing rate over different binwidths. Simpler patterned cells could be closely matched by single neuron models incorporating a hyperpolarising afterpotential and either a slow afterhyperpolarisation or a depolarising afterpotential, but many others could not. We then constructed network models with the challenge of explaining the more complex patterns. We assumed that neurons of a given type (with heterogeneity introduced by independently random patterns of external input) were mutually interconnected at random by excitatory synaptic connections (with a variable delay and a random chance of failure). Simple network models of one or two cell types were able to explain the more complex patterns. We then explored the information processing features of such networks that might be relevant for a decision-making network. We concluded that rhythm generation (in the slow theta range) and bistability arise as emergent properties of networks of heterogeneous VMN neurons.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Spontaneous discharge characteristic of neurons in the ventromedial nucleus of the rat hypothalamus in vivo.Eur J Neurosci. 2008 Aug;28(4):693-706. doi: 10.1111/j.1460-9568.2008.06389.x. Epub 2008 Jul 30. Eur J Neurosci. 2008. PMID: 18671740
-
Spike patterning in oxytocin neurons: Capturing physiological behaviour with Hodgkin-Huxley and integrate-and-fire models.PLoS One. 2017 Jul 6;12(7):e0180368. doi: 10.1371/journal.pone.0180368. eCollection 2017. PLoS One. 2017. PMID: 28683135 Free PMC article.
-
Medial cerebellar nucleus projects to feeding-related neurons in the ventromedial hypothalamic nucleus in rats.Brain Struct Funct. 2017 Mar;222(2):957-971. doi: 10.1007/s00429-016-1257-2. Epub 2016 Jun 24. Brain Struct Funct. 2017. PMID: 27342113
-
Reward-dependent learning in neuronal networks for planning and decision making.Prog Brain Res. 2000;126:217-29. doi: 10.1016/S0079-6123(00)26016-0. Prog Brain Res. 2000. PMID: 11105649 Review.
-
Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF).Diabetes Metab Res Rev. 2003 Sep-Oct;19(5):348-56. doi: 10.1002/dmrr.404. Diabetes Metab Res Rev. 2003. PMID: 12951642 Review.
Cited by
-
Sensory-thresholded switch of neural firing states in a computational model of the ventromedial hypothalamus.Front Comput Neurosci. 2022 Sep 7;16:964634. doi: 10.3389/fncom.2022.964634. eCollection 2022. Front Comput Neurosci. 2022. PMID: 36157840 Free PMC article.
-
Integrative Functions of the Hypothalamus: Linking Cognition, Emotion and Physiology for Well-being and Adaptability.Ann Neurosci. 2025 Apr;32(2):128-142. doi: 10.1177/09727531241255492. Epub 2024 Jun 12. Ann Neurosci. 2025. PMID: 39544638 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

