Targeting microglia with lentivirus and AAV: Recent advances and remaining challenges
- PMID: 31158432
- PMCID: PMC6734419
- DOI: 10.1016/j.neulet.2019.134310
Targeting microglia with lentivirus and AAV: Recent advances and remaining challenges
Abstract
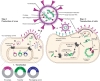
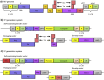
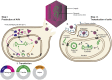
Microglia have emerged as a critical component of neurodegenerative diseases. Genetic manipulation of microglia can elucidate their functional impact in disease. In neuroscience, recombinant viruses such as lentiviruses and adeno-associated viruses (AAVs) have been successfully used to target various cell types in the brain, although effective transduction of microglia is rare. In this review, we provide a short background of lentiviruses and AAVs, and strategies for designing recombinant viral vectors. Then, we will summarize recent literature on successful microglial transductions in vitro and in vivo, and discuss the current challenges. Finally, we provide guidelines for reporting the efficiency and specificity of viral targeting in microglia, which will enable the microglial research community to assess and improve methodologies for future studies.
Keywords: Adeno-associated virus (AAV); Brain; In-vivo transduction; Lentivirus; Microglia.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23:1018–1027. - PubMed
-
- Kim Y.S., Joh T.H. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006;38:333–347. - PubMed
-
- Enquist L.W., Card J.P. Recent advances in the use of neurotropic viruses for circuit analysis. Curr. Opin. Neurobiol. 2003;13:603–606. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

