Enhanced tumor retention of NTSR1-targeted agents by employing a hydrophilic cysteine cathepsin inhibitor
- PMID: 31158752
- PMCID: PMC6636858
- DOI: 10.1016/j.ejmech.2019.05.068
Enhanced tumor retention of NTSR1-targeted agents by employing a hydrophilic cysteine cathepsin inhibitor
Abstract
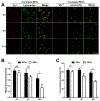
We explored the approach of using an analog of E-64, a well-known and hydrophilic cysteine cathepsin (CC) inhibitor, as a potent cysteine cathepsin-trapping agent (CCTA) to improve the tumor retention of low-molecular-weight, receptor-targeted radiopharmaceuticals. The synthesized hydrophilic CCTA-incorporated, NTSR1-targeted agents demonstrated a substantial increase in cellular retention upon uptake into the NTRS1-positive HT-29 human colon cancer cell line. Similarly, biodistribution studies using HT-29 xenograft mice revealed a significant and substantial increase in tumor retention for the CCTA-incorporated, NTSR1-targeted agent. The intracellular trapping mechanism of the CCTA-incorporated agents by macromolecular adduct formation was confirmed using multiple in vitro and in vivo techniques. Furthermore, utilization of the more hydrophilic CCTA greatly increased the hydrophilicity of the resulting NTSR1-targeted constructs leading to substantial decreases in most non-target tissues in contrast to our previously reported dipeptidyl acyloxymethyl ketone (AOMK) constructs. This work further confirms that the CCTA trapping approach can make significant improvements in the clinical potential of NTSR1-and other receptor-targeted radiopharmaceuticals.
Keywords: Cysteine cathepsin inhibitor; E−64 analogue; NTSR1; Radiopharmaceutical; Trapping agent; Tumor retention.
Copyright © 2019 Elsevier Masson SAS. All rights reserved.
Figures

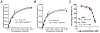


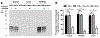


References
-
- Gambhir SS, Molecular imaging of cancer with positron emission tomography, Nat. Rev. Cancer 2 (2002) 683–693. - PubMed
-
- Okarvi S, Peptide-based radiopharmaceuticals: Future tools for diagnostic imaging of cancers and other diseases, Med. Res. Rev 24 (2004) 357–397. - PubMed
-
- Srinivasarao M, Galliford CV, Low PS, Principles in the design of ligand-targeted cancer therapeutics and imaging agents, Nat. Rev. Drug Discov 14 (2015) 203–219. - PubMed
-
- Okarvi S, Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer, Cancer Treat. Rev 34 (2008) 13–26. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

