Mapping human microbiome drug metabolism by gut bacteria and their genes
- PMID: 31158845
- PMCID: PMC6597290
- DOI: 10.1038/s41586-019-1291-3
Mapping human microbiome drug metabolism by gut bacteria and their genes
Abstract
Individuals vary widely in their responses to medicinal drugs, which can be dangerous and expensive owing to treatment delays and adverse effects. Although increasing evidence implicates the gut microbiome in this variability, the molecular mechanisms involved remain largely unknown. Here we show, by measuring the ability of 76 human gut bacteria from diverse clades to metabolize 271 orally administered drugs, that many drugs are chemically modified by microorganisms. We combined high-throughput genetic analyses with mass spectrometry to systematically identify microbial gene products that metabolize drugs. These microbiome-encoded enzymes can directly and substantially affect intestinal and systemic drug metabolism in mice, and can explain the drug-metabolizing activities of human gut bacteria and communities on the basis of their genomic contents. These causal links between the gene content and metabolic activities of the microbiota connect interpersonal variability in microbiomes to interpersonal differences in drug metabolism, which has implications for medical therapy and drug development across multiple disease indications.
Figures

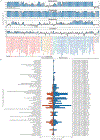














Comment in
-
Microbes make metabolic mischief by targeting drugs.Nature. 2019 Jun;570(7762):453-454. doi: 10.1038/d41586-019-01851-x. Nature. 2019. PMID: 31235939 No abstract available.
References
-
- Obach RS & Esbenshade TA Pharmacologically Active Drug Metabolites: Impact on Drug Discovery and Pharmacotherapy. Pharmacol Rev 65, 578–640 (2013). - PubMed
-
- Sousa T et al. On the colonic bacterial metabolism of azo-bonded prodrugsof 5-aminosalicylic acid. J Pharm Sci 103, 3171–3175 (2014). - PubMed
-
- Okuda H, Ogura K, Kato A, Takubo H & Watabe T A possible mechanism of eighteen patient deaths caused by interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. J Pharmacol Exp Ther 287, 791–799 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

