Detection of Epstein-Barr Virus Infection in Non-Small Cell Lung Cancer
- PMID: 31159203
- PMCID: PMC6627930
- DOI: 10.3390/cancers11060759
Detection of Epstein-Barr Virus Infection in Non-Small Cell Lung Cancer
Abstract
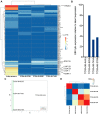
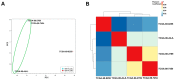
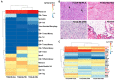
Previous investigations proposed a link between the Epstein-Barr virus (EBV) and lung cancer (LC), but the results are highly controversial largely due to the insufficient sample size and the inherent limitation of the traditional viral screening methods such as PCR. Unlike PCR, current next-generation sequencing (NGS) utilizes an unbiased method for the global assessment of all exogenous agents within a cancer sample with high sensitivity and specificity. In our current study, we aim to resolve this long-standing controversy by utilizing our unbiased NGS-based informatics approaches in conjunction with traditional molecular methods to investigate the role of EBV in a total of 1127 LC. In situ hybridization analysis of 110 LC and 10 normal lung samples detected EBV transcripts in 3 LC samples. Comprehensive virome analyses of RNA sequencing (RNA-seq) data sets from 1017 LC and 110 paired adjacent normal lung specimens revealed EBV transcripts in three lung squamous cell carcinoma and one lung adenocarcinoma samples. In the sample with the highest EBV coverage, transcripts from the BamHI A region accounted for the majority of EBV reads. Expression of EBNA-1, LMP-1 and LMP-2 was observed. A number of viral circular RNA candidates were also detected. Thus, we for the first time revealed a type II latency-like viral transcriptome in the setting of LC in vivo. The high-level expression of viral BamHI A transcripts in LC suggests a functional role of these transcripts, likely as long non-coding RNA. Analyses of cellular gene expression and stained tissue sections indicated an increased immune cell infiltration in the sample expressing high levels of EBV transcripts compared to samples expressing low EBV transcripts. Increased level of immune checkpoint blockade factors was also detected in the sample with higher levels of EBV transcripts, indicating an induced immune tolerance. Lastly, inhibition of immune pathways and activation of oncogenic pathways were detected in the sample with high EBV transcripts compared to the EBV-low LC indicating the direct regulation of cancer pathways by EBV. Taken together, our data support the notion that EBV likely plays a pathological role in a subset of LC.
Keywords: EBV; Epstein-Barr virus; NGS; NSCLC; next-generation sequencing; non-small cell lung cancer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures





References
-
- Roy A., Dey S., Chatterjee R. Prevalence of serum IgG and IgM antibodies against Epstein-Barr virus capsid antigen in indian patients with respiratory tract carcinomas. Neoplasma. 1994;41:29–33. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

