Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine
- PMID: 31159216
- PMCID: PMC6600135
- DOI: 10.3390/ijms20112688
Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine
Abstract
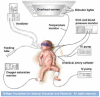
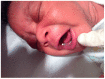
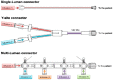
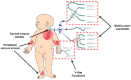
The development of age-appropriate formulations should focus on dosage forms that can deliver variable yet accurate doses that are safe and acceptable to the child, are matched to his/her development and ability, and avoid medication errors. However, in the past decade, the medication needs of neonates have largely been neglected. The aim of this review is to expand on what differentiates the needs of preterm and term neonates from those of the older paediatric subsets, in terms of environment of care, ability to measure and administer the dose (from the perspective of the patient and carer, the routes of administration, the device and the product), neonatal biopharmaceutics and regulatory challenges. This review offers insight into those challenges posed by the formulation of medicinal products for neonatal patients in order to support the development of clinically relevant products.
Keywords: NICU; administration; biopharmaceutics; device; dosage form; excipient; formulation; formulation development; inhaled; intra nasal; medication error; neonates; oral; parenteral; product development; topical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- European Medicines Agency Ich e11(r1) Guideline on Clinical Investigation of Medicinal Products in the Pediatric Population. [(accessed on 19 March 2019)]; Available online: https://www.ema.europa.eu/documents/scientific-guideline/ich-e11r1-guide....
-
- March of Dimes. PMNCH. Save the Children. WHO Born too Soon: The Global Action Report on Preterm Birth. [(accessed on 19 March 2019)]; Available online: https://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf.
-
- Allegaert K., Samardzic J., Bajcetic M., van den Anker J.N. Developmental pharmacology and therapeutics in neonatal medicine. In: Buonocore G., Bracci R., Weindling M., editors. Neonatology: A Practical Approach to Neonatal Diseases. 2nd ed. Springer; Cham, Switzerland: 2018. pp. 693–707.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

