Luteolin-7- O-β-d-Glucoside Inhibits Cellular Energy Production Interacting with HEK2 in Keratinocytes
- PMID: 31159225
- PMCID: PMC6600217
- DOI: 10.3390/ijms20112689
Luteolin-7- O-β-d-Glucoside Inhibits Cellular Energy Production Interacting with HEK2 in Keratinocytes
Abstract
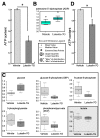
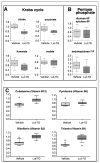
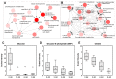
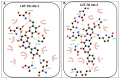
Flavonoids have been demonstrated to affect the activity of many mammalian enzyme systems. Their functional phenolic groups are able to mediate antioxidant effects by scavenging free radicals. Molecules of this class have been found able to modulate the activity of kinases, phospholipase A2, cyclooxygenases, lipoxygenase, glutathione S-transferase, and many others. Recently, it has been demonstrated that luteolin, in the form of Luteolin-7-O-β-d-glucoside (LUT-7G) is able to induce the keratinocyte differentiation process in vitro. This flavonoid is able to counteract the proliferative effects of IL-22/IL6 pathway by the inhibition of STAT3 activity also in vivo in a psoriatic mouse model. Observations on energy metabolism changes of differentiating cells led us to perform a complete metabolomics analysis using human primary keratinocytes treated with LUT-7G. Our results show that LUT-7G, is not only able to impair the nuclear translocation of STAT3, but it also blocks the energy metabolism pathway, depressing the glycolytic and Krebs pathway by the inhibition of hexokinase 2 activity. These data confirm that LUT-7G can be proposed as a potential candidate for the treatment of inflammatory and proliferative diseases, but its role as a hexokinase 2 (HEK2) inhibitor opens new perspectives in nutritional science, and especially in cancer therapy, in which the inhibition of the Warburg effect could be relevant.
Keywords: glycolysis; hexokinase inhibitor; luteolin-7-O-β-d-glucoside.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

