Human Enriched Serum Following Hydrolysed Collagen Absorption Modulates Bone Cell Activity: from Bedside to Bench and Vice Versa
- PMID: 31159319
- PMCID: PMC6627680
- DOI: 10.3390/nu11061249
Human Enriched Serum Following Hydrolysed Collagen Absorption Modulates Bone Cell Activity: from Bedside to Bench and Vice Versa
Abstract
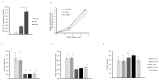
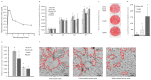
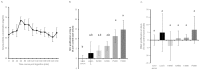
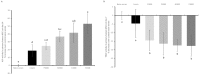
Collagen proteins are crucial components of the bone matrix. Since collagen-derived products are widely used in the food and supplement industry, one may raise the question whether collagen-enriched diets can provide benefits for the skeleton. In this study, we designed an innovative approach to investigate this question taking into account the metabolites that are formed by the digestive tract and appear in the circulation after ingestion of hydrolysed collagen. Blood samples collected in clinical and pre-clinical trials following ingestion and absorption of hydrolysed collagen were processed and applied on bone-related primary cell cultures. This original ex vivo methodology revealed that hydrolysed collagen-enriched serum had a direct impact on the behaviour of cells from both human and mouse origin that was not observed with controls (bovine serum albumin or hydrolysed casein-enriched serum). These ex vivo findings were fully in line with in vivo results obtained from a mouse model of post-menopausal osteoporosis. A significant reduction of bone loss was observed in mice supplemented with hydrolysed collagen compared to a control protein. Both the modulation of osteoblast and osteoclast activity observed upon incubation with human or mouse serum ex vivo and the attenuation of bone loss in vivo, clearly indicates that the benefits of hydrolysed collagen for osteoporosis prevention go beyond the effect of a simple protein supplementation.
Keywords: absorption; bone; collagen peptides; hydrolysed collagen; metabolites; nutrition; osteoporosis.
Conflict of interest statement
Fabien Wauquier, Henri Granel, Audrey Daneault, Gael Rochefort, Jérome Guicheux, Adeline Blot, Nathalie Meunier and Yohann Wittrant have no conflict of interest to declare. Janne Prawitt and Véronique Fabien-Soulé work for Rousselot and provided the hydrolysed collagens.
Figures
Similar articles
-
Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: an in vitro and in vivo study.Bone. 2010 Mar;46(3):827-34. doi: 10.1016/j.bone.2009.10.035. Epub 2009 Nov 4. Bone. 2010. PMID: 19895915
-
The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss.J Nutr Biochem. 2011 Jan;22(1):8-15. doi: 10.1016/j.jnutbio.2009.11.002. Epub 2010 Mar 16. J Nutr Biochem. 2011. PMID: 20233653
-
Bovine lactoferrin improves bone status of ovariectomized mice via immune function modulation.Bone. 2011 May 1;48(5):1028-35. doi: 10.1016/j.bone.2011.02.002. Epub 2011 Feb 16. Bone. 2011. PMID: 21303707
-
Anti-osteoporotic activity of harpagide by regulation of bone formation in osteoblast cell culture and ovariectomy-induced bone loss mouse models.J Ethnopharmacol. 2016 Feb 17;179:66-75. doi: 10.1016/j.jep.2015.12.025. Epub 2015 Dec 19. J Ethnopharmacol. 2016. PMID: 26712566
-
[An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing].Nutr Hosp. 2015 Jul 18;32 Suppl 1:62-6. doi: 10.3305/nh.2015.32.sup1.9482. Nutr Hosp. 2015. PMID: 26267777 Review. Spanish.
Cited by
-
Effect of a Daily Collagen Peptide Supplement on Digestive Symptoms in Healthy Women: 2-Phase Mixed Methods Study.JMIR Form Res. 2022 May 31;6(5):e36339. doi: 10.2196/36339. JMIR Form Res. 2022. PMID: 35639457 Free PMC article.
-
Benefits of Circulating Human Metabolites from Fish Cartilage Hydrolysate on Primary Human Dermal Fibroblasts, an Ex Vivo Clinical Investigation for Skin Health Applications.Nutrients. 2022 Nov 25;14(23):5027. doi: 10.3390/nu14235027. Nutrients. 2022. PMID: 36501057 Free PMC article.
-
Reduced Production of Pro-Inflammatory and Pro-Catabolic Factors by Human Serum Metabolites Derived from a Patented Saffron Extract Intake.Pharmaceutics. 2024 Feb 28;16(3):336. doi: 10.3390/pharmaceutics16030336. Pharmaceutics. 2024. PMID: 38543230 Free PMC article.
-
Non-targeted and targeted analysis of collagen hydrolysates during the course of digestion and absorption.Anal Bioanal Chem. 2020 Feb;412(4):973-982. doi: 10.1007/s00216-019-02323-x. Epub 2019 Dec 24. Anal Bioanal Chem. 2020. PMID: 31872275 Free PMC article.
-
Circulating Human Serum Metabolites Derived from the Intake of a Saffron Extract (Safr'InsideTM) Protect Neurons from Oxidative Stress: Consideration for Depressive Disorders.Nutrients. 2022 Apr 5;14(7):1511. doi: 10.3390/nu14071511. Nutrients. 2022. PMID: 35406124 Free PMC article.
References
-
- Hanley D.A., McClung M.R., Davison K.S., Dian L., Harris S.T., Miller P.D., Lewiecki E.M., Kendler D.L. Western osteoporosis alliance clinical practice series: Evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am. J. Med. 2017;130:862-e1. doi: 10.1016/j.amjmed.2017.03.002. - DOI - PubMed
-
- Elam M.L., Elam M.L., Johnson S.A., Hooshmand S., Feresin R.G., Payton M.E., Gu J., Arjmandi B.H. A calcium-collagen chelate dietary supplement attenuates bone loss in postmenopausal women with osteopenia: A randomized controlled trial. J. Med. Food. 2015;18:324–331. doi: 10.1089/jmf.2014.0100. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

