Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy
- PMID: 31159345
- PMCID: PMC6600381
- DOI: 10.3390/ijms20112698
Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy
Abstract
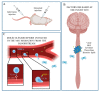
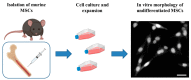
Spinal cord injury (SCI) constitutes an inestimable public health issue. The most crucial phase in the pathophysiological process of SCI concerns the well-known secondary injury, which is the uncontrolled and destructive cascade occurring later with aberrant molecular signaling, inflammation, vascular changes, and secondary cellular dysfunctions. The use of mesenchymal stem cells (MSCs) represents one of the most important and promising tested strategies. Their appeal, among the other sources and types of stem cells, increased because of their ease of isolation/preservation and their properties. Nevertheless, encouraging promise from preclinical studies was followed by weak and conflicting results in clinical trials. In this review, the therapeutic role of MSCs is discussed, together with their properties, application, limitations, and future perspectives.
Keywords: mesenchymal stem cells; regenerative medicine; spinal cord injury; translational medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- International Perspectives on Spinal Cord Injury. [(accessed on 31 October 2014)]; Available online: http://apps.who.int/iris/bitstream/10665/94190/1/9789241564663_eng.pdf.
-
- Wilson J.R., Tetreault L.A., Kwon B.K., Arnold P.M., Mroz T.E., Shaffrey C., Harrop J.S., Chapman J.R., Casha S., Skelly A.C., et al. Timing of Decompression in Patients with Acute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017;7:95S–115S. doi: 10.1177/2192568217701716. - DOI - PMC - PubMed

