In Vitro Studies Reveal Antiurolithic Effect of Antioxidant Sulfated Polysaccharides from the Green Seaweed Caulerpa cupressoides var flabellata
- PMID: 31159355
- PMCID: PMC6628234
- DOI: 10.3390/md17060326
In Vitro Studies Reveal Antiurolithic Effect of Antioxidant Sulfated Polysaccharides from the Green Seaweed Caulerpa cupressoides var flabellata
Abstract
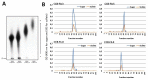
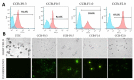
Urolithiasis affects approximately 10% of the world population and is strongly associated with calcium oxalate (CaOx) crystals. Currently, there is no efficient compound that can be used to prevent this disease. However, seaweeds' sulfated polysaccharides (SPs) can change the CaOx crystals surface's charge and thus modify the crystallization dynamics, due to the interaction of the negative charges of these polymers with the crystal surface during their synthesis. We observed that the SPs of Caulerpa cupressoides modified the morphology, size and surface charge of CaOx crystals. Thus, these crystals became similar to those found in healthy persons. In the presence of SPs, dihydrate CaOx crystals showed rounded or dumbbell morphology. Infrared analysis, fluorescence microscopy, flow cytometry (FITC-conjugated SPs) and atomic composition analysis (EDS) allowed us to propose the mode of action between the Caulerpa's SPs and the CaOx crystals. This study is the first step in understanding the interactions between SPs, which are promising molecules for the treatment of urolithiasis, and CaOx crystals, which are the main cause of kidney stones.
Keywords: COD dumbbell; COD stabilization; green algae; urolithiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Sofia N.H., Walter T.M., Sanatorium T. Prevalence and risk factors of kidney stone. GJRA. 2016;5:183–187.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

