Slowing Down Ageing: The Role of Nutrients and Microbiota in Modulation of the Epigenome
- PMID: 31159371
- PMCID: PMC6628342
- DOI: 10.3390/nu11061251
Slowing Down Ageing: The Role of Nutrients and Microbiota in Modulation of the Epigenome
Abstract
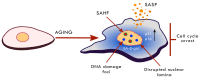
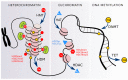
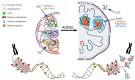
The human population is getting ageing. Both ageing and age-related diseases are correlated with an increased number of senescent cells in the organism. Senescent cells do not divide but are metabolically active and influence their environment by secreting many proteins due to a phenomenon known as senescence associated secretory phenotype (SASP). Senescent cells differ from young cells by several features. They possess more damaged DNA, more impaired mitochondria and an increased level of free radicals that cause the oxidation of macromolecules. However, not only biochemical and structural changes are related to senescence. Senescent cells have an altered chromatin structure, and in consequence, altered gene expression. With age, the level of heterochromatin decreases, and less condensed chromatin is more prone to DNA damage. On the one hand, some gene promoters are easily available for the transcriptional machinery; on the other hand, some genes are more protected (locally increased level of heterochromatin). The structure of chromatin is precisely regulated by the epigenetic modification of DNA and posttranslational modification of histones. The methylation of DNA inhibits transcription, histone methylation mostly leads to a more condensed chromatin structure (with some exceptions) and acetylation plays an opposing role. The modification of both DNA and histones is regulated by factors present in the diet. This means that compounds contained in daily food can alter gene expression and protect cells from senescence, and therefore protect the organism from ageing. An opinion prevailed for some time that compounds from the diet do not act through direct regulation of the processes in the organism but through modification of the physiology of the microbiome. In this review we try to explain the role of some food compounds, which by acting on the epigenetic level might protect the organism from age-related diseases and slow down ageing. We also try to shed some light on the role of microbiome in this process.
Keywords: ageing; epigenetic; microbiome; nutrition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

