Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model
- PMID: 31159860
- PMCID: PMC6547527
- DOI: 10.1186/s40168-019-0701-y
Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model
Abstract
Background: The development of the rumen is an important physiological challenge for young ruminants. Previous studies have shown that starter feeding can effectively facilitate the growth and development of the rumen in ruminants. However, the mechanism through which starter feeding stimulates the development of the rumen is not clear. Here, we performed an integrated analysis in ruminal microbiota and a host transcriptomic profile in a lamb model with the intervention of starter feed to understand the ruminal microbiome-host crosstalk in stimulating the development of the ruminal epithelium.
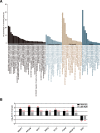
Results: Decreased ruminal pH and increased acetate and butyrate concentrations in the rumen, followed by increasing rumen organ index, were observed in lambs supplemented with starter. Using metagenome sequencing in combination with 16S rRNA and 18S rRNA gene amplicon sequencing, the results showed the abundance of acetate-producing Mitsuokella spp., lactate-producing Sharpea spp., lactate-utilizing Megasphaera spp., and Entodinium spp. was enriched in rumen microbial communities in the starter-feed group. The abundances of genes involved in sugar degradation were decreased in starter-feed lambs, but the GH13 encoding α-amylase was obviously increased. Rumen epithelial transcriptome analysis revealed that seven differentially expressed genes, including MAPK1, PIK3CB, TNFSF10, ITGA6, SNAI2, SAV1, and DLG, related to the cell growth module were upregulated, and BAD's promotion of cell death was downregulated. Correlation analysis revealed that the increase in the concentrations of acetate and butyrate significantly correlated with the expression of these genes, which indicates acetate and butyrate likely acted as important drivers in the ruminal microbiome-host crosstalk.
Conclusions: The present study comprehensively describes the symbiotic relationship between the rumen microbiota and the host in lambs after starter feeding. Our data demonstrates that the microbiome-driven generation of acetate and butyrate mediated the growth-related genes' regulation of the growth-associated signalling pathway in the ruminal epithelium. These co-development networks regulated many physiological processes in the epithelium, including papillae morphology and rumen epithelial growth.
Keywords: Crosstalk; Lamb; Rumen; Starter feeding; Symbiotic microbiome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

