The DM-scope registry: a rare disease innovative framework bridging the gap between research and medical care
- PMID: 31159885
- PMCID: PMC6547518
- DOI: 10.1186/s13023-019-1088-3
The DM-scope registry: a rare disease innovative framework bridging the gap between research and medical care
Abstract
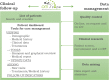
Background: The relevance of registries as a key component for developing clinical research for rare diseases (RD) and improving patient care has been acknowledged by most stakeholders. As recent studies pointed to several limitations of RD registries our challenge was (1) to improve standardization and data comparability; (2) to facilitate interoperability between existing RD registries; (3) to limit the amount of incomplete data; (4) to improve data quality. This report describes the innovative concept of the DM-Scope Registry that was developed to achieve these objectives for Myotonic Dystrophy (DM), a prototypical example of highly heterogeneous RD. By the setting up of an integrated platform attractive for practitioners use, we aimed to promote DM epidemiology, clinical research and patients care management simultaneously.
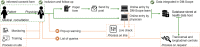
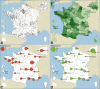
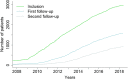
Results: The DM-Scope Registry is a result of the collaboration within the French excellence network established by the National plan for RDs. Inclusion criteria is all genetically confirmed DM individuals, independently of disease age of onset. The dataset includes social-demographic data, clinical features, genotype, and biomaterial data, and is adjustable for clinical trial data collection. To date, the registry has a nationwide coverage, composed of 55 neuromuscular centres, encompassing the whole disease clinical and genetic spectrum. This widely used platform gathers almost 3000 DM patients (DM1 n = 2828, DM2 n = 142), both children (n = 322) and adults (n = 2648), which accounts for > 20% of overall registered DM patients internationally. The registry supported 10 research studies of various type i.e. observational, basic science studies and patient recruitment for clinical trials.
Conclusion: The DM-Scope registry represents the largest collection of standardized data for the DM population. Our concept improved collaboration among health care professionals by providing annual follow-up of quality longitudinal data collection. The combination of clinical features and biomolecular materials provides a comprehensive view of the disease in a given population. DM-Scope registry proves to be a powerful device for promoting both research and medical care that is suitable to other countries. In the context of emerging therapies, such integrated platform contributes to the standardisation of international DM research and for the design of multicentre clinical trials. Finally, this valuable model is applicable to other RDs.
Keywords: Medical care; Myotonic dystrophy; Platform; Rare disease registry; Research.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Council recommendation on an action in the field of rare diseases. 2009. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:000...
-
- European Union Committee of experts on Rare Diseases Recommendations on quality criteria for centers of expertise for rare diseases in Member states. 2011 http://eucerd.eu/?post_type=document&p=1224
-
- European Union Committee of Experts on Rare diseases: European Union Committee of Experts on rare diseases: recommendations on European reference Networks for rare diseases. 2013 http://www.eucerd.eu/?post_type=document&p=2207
-
- Dawkins HJS, Draghia-Akli R, Lasko P, Lau LPL, Jonker AH, Cutillo CM, Rath A, Boycott KM, Baynam G, Lochmüller H, Kaufmann P, Le Cam Y, Hivert V. Austin CP; international rare diseases research consortium (IRDiRC). Progress in rare diseases research 2010-2016: an IRDiRC perspective. Clin Transl Sci. 2018;11(1):11–20. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

