O-acetylation of typhoid capsular polysaccharide confers polysaccharide rigidity and immunodominance by masking additional epitopes
- PMID: 31160100
- PMCID: PMC6997886
- DOI: 10.1016/j.vaccine.2019.05.050
O-acetylation of typhoid capsular polysaccharide confers polysaccharide rigidity and immunodominance by masking additional epitopes
Abstract
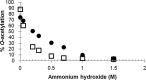
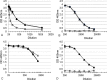
In this work, we explore the effects of O-acetylation on the physical and immunological characteristics of the WHO International Standards of Vi polysaccharide (Vi) from both Citrobacter freundii and Salmonella enterica serovar Typhi. We find that, although structurally identical according to NMR, the two Vi standards have differences with respect to susceptibility to de-O-acetylation and viscosity in water. Vi standards from both species have equivalent mass and O-acetylation-dependent binding to a mouse monoclonal antibody and to anti-Vi polyclonal antisera, including the WHO International Standard for human anti-typhoid capsular Vi PS IgG. This study also confirms that human anti-Vi sera binds to completely de-O-acetylated Vi. Molecular dynamics simulations provide conformational rationales for the known effect of de-O-acetylation both on the viscosity and antigenicity of the Vi, demonstrating that de-O-acetylation has a very marked effect on the conformation and dynamic behavior of the Vi, changing the capsular polysaccharide from a rigid helix into a more flexible coil, as well as enhancing the strong interaction of the polysaccharide with sodium ions. Partial de-O-acetylation of Vi revealed hidden epitopes that were recognized by human and sheep anti-Vi PS immune sera. These findings have significance for the manufacture and evaluation of Vi vaccines.
Keywords: Enteric; Glycoconjugate; Molecular modelling; Nuclear magnetic resonance; Vaccine; Vi polysaccharide.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Authors from NIBSC have none to declare.
Figures

 ), N-acetyl (
), N-acetyl ( ), O-acetyl (
), O-acetyl ( ), and carboxyl (
), and carboxyl ( ).
).



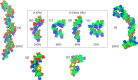
 ; O-acetyl,
; O-acetyl,  ; carboxyl,
; carboxyl,  , with sodium ions,
, with sodium ions,  and the remaining atoms colored
and the remaining atoms colored  .
.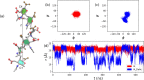
 ; O-acetyl,
; O-acetyl,  ; carboxyl,
; carboxyl,  . Simulation time series of the φ and ψ dihedral angles show differing conformations for the glycosidic linkage in (b) O-acetylated Vi and (c) de-O-acetylated Vi, with the de-O-acetylated strand rotating more freely (d). The same is true for the chain extension: the r end-to-end distance time series is relatively constant for O-acetylated Vi (
. Simulation time series of the φ and ψ dihedral angles show differing conformations for the glycosidic linkage in (b) O-acetylated Vi and (c) de-O-acetylated Vi, with the de-O-acetylated strand rotating more freely (d). The same is true for the chain extension: the r end-to-end distance time series is relatively constant for O-acetylated Vi ( ) and much more variable in de-O-acetylated Vi (
) and much more variable in de-O-acetylated Vi ( ).
).References
-
- Felix A., Pitt R.M. A new antigen of B. typhosus. Its relation to virulence and to active and passive immunisation. Lancet. 1934;224(5787):186–191.
-
- Robbins J.D., Robbins J.B. Re-examination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984;150(3):436–449. PMID: 6207249. - PubMed
-
- Clark W.R., McLaughlin J., Webster M.E. An aminohexuronic acid as the principal hydrolytic component of the Vi antigen. J Biol Chem. 1958;230(1):81–89. PMID: 13502376. - PubMed
-
- Heyns K., Kiessling G. Strukturaufklärung des Vi-antigens aus Citrobacter freundii (E. coli) 5396/38. Carb Res. 1967;3(3):340–353.
-
- Bystricky S., Szu S.C. O-acetylation affects the binding properties of the carboxyl group on the Vi bacterial polysaccharide. Biophys Chem. 1994;51(1):1–7. PMID: 8061223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

