Neutrophil Diversity in Health and Disease
- PMID: 31160207
- PMCID: PMC7185435
- DOI: 10.1016/j.it.2019.04.012
Neutrophil Diversity in Health and Disease
Abstract
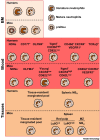
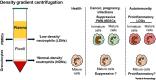
New evidence has challenged the outdated dogma that neutrophils are a homogeneous population of short-lived cells. Although neutrophil subpopulations with distinct functions have been reported under homeostatic and pathological conditions, a full understanding of neutrophil heterogeneity and plasticity is currently lacking. We review here current knowledge of neutrophil heterogeneity and diversity, highlighting the need for deep genomic, phenotypic, and functional profiling of the identified neutrophil subpopulations to determine whether these cells truly represent bona fide novel neutrophil subsets. We suggest that progress in understanding neutrophil heterogeneity will allow the identification of clinically relevant neutrophil subpopulations that may be used in the diagnosis of specific diseases and lead to the development of new therapeutic approaches.
Keywords: immune regulation; neutrophils; subpopulations.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures



References
-
- Scapini P. Granulocytes and mast cells. In: Paul W., editor. Fundamental Immunology. 7th edn. Wolters Kluwer-Lippincott Williams & Wilkins; 2013. pp. 468–486.
-
- Ley K. Neutrophils: new insights and open questions. Sci. Immunol. 2018;3 - PubMed
-
- Ng L.G. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019;19:255–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

