The endoderm: a divergent cell lineage with many commonalities
- PMID: 31160415
- PMCID: PMC6589075
- DOI: 10.1242/dev.150920
The endoderm: a divergent cell lineage with many commonalities
Abstract
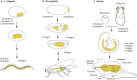
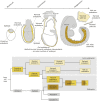
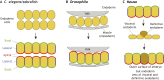
The endoderm is a progenitor tissue that, in humans, gives rise to the majority of internal organs. Over the past few decades, genetic studies have identified many of the upstream signals specifying endoderm identity in different model systems, revealing them to be divergent from invertebrates to vertebrates. However, more recent studies of the cell behaviours driving endodermal morphogenesis have revealed a surprising number of shared features, including cells undergoing epithelial-to-mesenchymal transitions (EMTs), collective cell migration, and mesenchymal-to-epithelial transitions (METs). In this Review, we highlight how cross-organismal studies of endoderm morphogenesis provide a useful perspective that can move our understanding of this fascinating tissue forward.
Keywords: Collective cell migration; Endoderm; Epithelial-to-mesenchymal transitions; Mesenchymal-to-epithelial transitions; Morphogenesis.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

