Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells
- PMID: 31160463
- PMCID: PMC6589653
- DOI: 10.1073/pnas.1900172116
Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells
Abstract
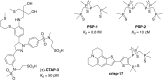
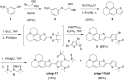
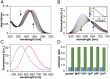
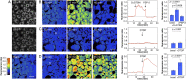
Copper is controlled by a sophisticated network of transport and storage proteins within mammalian cells, yet its uptake and efflux occur with rapid kinetics. Present as Cu(I) within the reducing intracellular environment, the nature of this labile copper pool remains elusive. While glutathione is involved in copper homeostasis and has been assumed to buffer intracellular copper, we demonstrate with a ratiometric fluorescent indicator, crisp-17, that cytosolic Cu(I) levels are buffered to the vicinity of 1 aM, where negligible complexation by glutathione is expected. Enabled by our phosphine sulfide-stabilized phosphine (PSP) ligand design strategy, crisp-17 offers a Cu(I) dissociation constant of 8 aM, thus exceeding the binding affinities of previous synthetic Cu(I) probes by four to six orders of magnitude. Two-photon excitation microscopy with crisp-17 revealed rapid, reversible increases in intracellular Cu(I) availability upon addition of the ionophoric complex CuGTSM or the thiol-selective oxidant 2,2'-dithiodipyridine (DTDP). While the latter effect was dramatically enhanced in 3T3 cells grown in the presence of supplemental copper and in cultured Menkes mutant fibroblasts exhibiting impaired copper efflux, basal Cu(I) availability in these cells showed little difference from controls, despite large increases in total copper content. Intracellular copper is thus tightly buffered by endogenous thiol ligands with significantly higher affinity than glutathione. The dual utility of crisp-17 to detect normal intracellular buffered Cu(I) levels as well as to probe the depth of the labile copper pool in conjunction with DTDP provides a promising strategy to characterize perturbations of cellular copper homeostasis.
Keywords: Menkes disease; copper homeostasis; fluorescent probes; glutathione; two-photon microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Öhrvik H., Aaseth J., Horn N., Orchestration of dynamic copper navigation—new and missing pieces. Metallomics 9, 1204–1229 (2017). - PubMed
-
- Morgan M. T., Bagchi P., Fahrni C. J., “Fluorescent probes for monovalent copper” in Metals in Cells, Culotta V, Scott RA, Eds. (Wiley, 2013), pp. 65–84.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

