Activated Peyer's patch B cells sample antigen directly from M cells in the subepithelial dome
- PMID: 31160559
- PMCID: PMC6547658
- DOI: 10.1038/s41467-019-10144-w
Activated Peyer's patch B cells sample antigen directly from M cells in the subepithelial dome
Abstract
The germinal center (GC) reaction in Peyer's patches (PP) requires continuous access to antigens, but how this is achieved is not known. Here we show that activated antigen-specific CCR6+CCR1+GL7- B cells make close contact with M cells in the subepithelial dome (SED). Using in situ photoactivation analysis of antigen-specific SED B cells, we find migration of cells towards the GC. Following antigen injection into ligated intestinal loops containing PPs, 40% of antigen-specific SED B cells bind antigen within 2 h, whereas unspecifc cells do not, indicating B cell-receptor involvment. Antigen-loading is not observed in M cell-deficient mice, but is unperturbed in mice depleted of classical dendritic cells (DC). Thus, we report a M cell-B cell antigen-specific transporting pathway in PP that is independent of DC. We propose that this antigen transporting pathway has a critical role in gut IgA responses, and should be taken into account when developing mucosal vaccines.
Conflict of interest statement
The authors declare no competing interests.
Figures
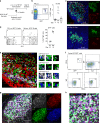

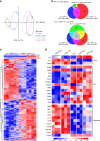
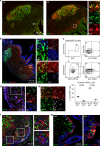
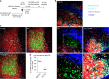
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

