Feed-forward regulation adaptively evolves via dynamics rather than topology when there is intrinsic noise
- PMID: 31160574
- PMCID: PMC6546794
- DOI: 10.1038/s41467-019-10388-6
Feed-forward regulation adaptively evolves via dynamics rather than topology when there is intrinsic noise
Abstract
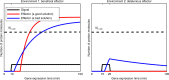
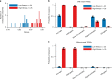
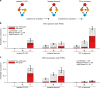
In transcriptional regulatory networks (TRNs), a canonical 3-node feed-forward loop (FFL) is hypothesized to evolve to filter out short spurious signals. We test this adaptive hypothesis against a novel null evolutionary model. Our mutational model captures the intrinsically high prevalence of weak affinity transcription factor binding sites. We also capture stochasticity and delays in gene expression that distort external signals and intrinsically generate noise. Functional FFLs evolve readily under selection for the hypothesized function but not in negative controls. Interestingly, a 4-node "diamond" motif also emerges as a short spurious signal filter. The diamond uses expression dynamics rather than path length to provide fast and slow pathways. When there is no idealized external spurious signal to filter out, but only internally generated noise, only the diamond and not the FFL evolves. While our results support the adaptive hypothesis, we also show that non-adaptive factors, including the intrinsic expression dynamics, matter.
Conflict of interest statement
The authors declare no competing interests.
Figures

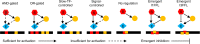
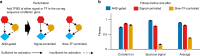



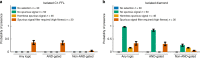
References
Publication types
MeSH terms
Substances
Grants and funding
- R35 GM118170/GM/NIGMS NIH HHS/United States
- R35GM118170/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- R01GM076041/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- 39667/John Templeton Foundation (JTF)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

