Stable cyclic (alkyl)(amino)carbene (cAAC) radicals with main group substituents
- PMID: 31160949
- PMCID: PMC6510188
- DOI: 10.1039/c9sc01351b
Stable cyclic (alkyl)(amino)carbene (cAAC) radicals with main group substituents
Abstract
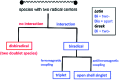
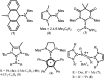
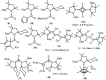
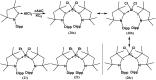
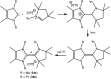
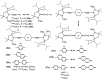
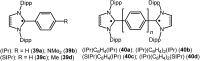
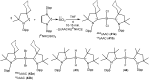
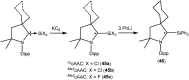
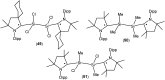
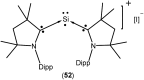
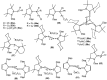
Isolation and characterization of stable radicals has been a long-pursued quest. While there has been some progress in this field particularly with respect to carbon, radicals involving heavier p-block elements are still considerably sparse. In this review we describe our recent successful efforts on the isolation of stable p-block element radicals particularly those involving aluminum, silicon, and phosphorus.
Figures

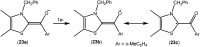
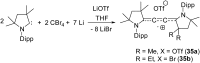
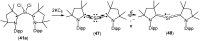
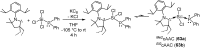
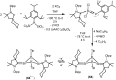
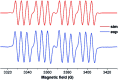
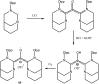




References
-
- Stable Radicals: Fundamental and Applied Aspects of Odd-Electron Compounds, ed. R. G. Hicks, Wiley, Chichester, 2010.
-
- Fossey J., Lefort D. and Serba J., Free Radicals in Organic Chemistry, John Wiley and Sons, 1995.
-
- Renaud P. and Sibi M. P., Radicals in Organic Synthesis, Wiley-VCH, 2008.
-
- Chatgilialoglu C. and Studer A., Encyclopedia of Radicals in Chemistry, Biology and Materials, Wiley-VCH, 2012.
-
- Power P. P. Chem. Rev. 2003;103:789–810. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

