Differential gene expression in relation to mating system in Peromyscine rodents
- PMID: 31161013
- PMCID: PMC6540711
- DOI: 10.1002/ece3.5181
Differential gene expression in relation to mating system in Peromyscine rodents
Abstract
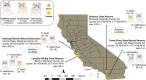
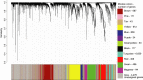
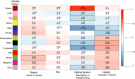
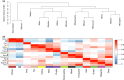
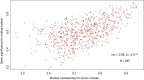
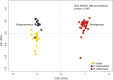
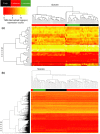
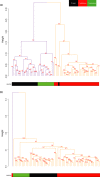
Behaviors that increase an individual's exposure to pathogens are expected to have important effects on immunoactivity. Because sexual reproduction typically requires close contact among conspecifics, mating systems provide an ideal opportunity to study the immunogenetic correlates of behaviors with high versus low risks of pathogen exposure. Despite logical links between polygynandrous mating behavior, increased pathogen exposure, and greater immunoactivity, these relationships have seldom been examined in nonhuman vertebrates. To explore interactions among these variables in a different lineage of mammals, we used RNAseq to study the gene expression profiles of liver tissue-a highly immunoactive organ-from sympatric populations of the monogamous California mouse (Peromyscus californicus) and two polygynandrous congeners (P. maniculatus and P. boylii). Differential expression and co-expression analyses revealed distinct patterns of gene activity among species, with much of this variation associated with differences in mating system. This tendency was particularly pronounced for MHC genes, with multiple MHC Class I genes being upregulated in the two polygynandrous species, as expected if exposure to sexually transmitted pathogens varies with mating system. Our results underscore the role of mating behavior in influencing patterns of gene expression and highlight the use of emerging transcriptomic tools in behavioral studies of free-living animals.
Keywords: gene expression; mating systems; monogamy; peromyscus; promiscuity; transcriptomics.
Figures

Similar articles
-
Mating system is correlated with immunogenetic diversity in sympatric species of Peromyscine mice.PLoS One. 2020 Jul 23;15(7):e0236084. doi: 10.1371/journal.pone.0236084. eCollection 2020. PLoS One. 2020. PMID: 32701975 Free PMC article.
-
Is promiscuity associated with enhanced selection on MHC-DQα in mice (genus Peromyscus)?PLoS One. 2012;7(5):e37562. doi: 10.1371/journal.pone.0037562. Epub 2012 May 23. PLoS One. 2012. PMID: 22649541 Free PMC article.
-
Genomic analysis of MHC-based mate choice in the monogamous California mouse.Behav Ecol. 2018 Sep-Oct;29(5):1167-1180. doi: 10.1093/beheco/ary096. Epub 2018 Jul 12. Behav Ecol. 2018. PMID: 30214134 Free PMC article.
-
Mammalian mating systems.Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):339-72. doi: 10.1098/rspb.1989.0027. Proc R Soc Lond B Biol Sci. 1989. PMID: 2567517 Review.
-
Monogamy without parental care? Social and genetic mating systems of avian brood parasites.Philos Trans R Soc Lond B Biol Sci. 2019 Apr 1;374(1769):20180201. doi: 10.1098/rstb.2018.0201. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30967092 Free PMC article. Review.
Cited by
-
Mating system is correlated with immunogenetic diversity in sympatric species of Peromyscine mice.PLoS One. 2020 Jul 23;15(7):e0236084. doi: 10.1371/journal.pone.0236084. eCollection 2020. PLoS One. 2020. PMID: 32701975 Free PMC article.
-
Mating system variation and gene expression in the male reproductive tract of Peromyscus mice.Mol Ecol. 2025 Aug;34(15):e17433. doi: 10.1111/mec.17433. Epub 2024 Jun 21. Mol Ecol. 2025. PMID: 39031829 Free PMC article.
References
-
- Bengston, S. E. , Dahan, R. A. , Donaldson, Z. , Phelps, S. M. , van Oers, K. , Sih, A. , & Bell, A. M. (2018). Genomic tools for behavioural ecologists to understand repeatable individual differences in behaviour. Nature Ecology & Evolution, 2(6), 944–955. 10.1038/s41559-017-0411-4 - DOI - PMC - PubMed
-
- Bessa‐Gomes, C. , Legendre, S. , & Clobert, J. (2004). Allee effects, mating systems and the extinction risk in populations with two sexes: Allee effects, mating systems and the extinction risk. Ecology Letters, 7(9), 802–812. 10.1111/j.1461-0248.2004.00632.x - DOI
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

