Beta-cell excitability and excitability-driven diabetes in adult Zebrafish islets
- PMID: 31161721
- PMCID: PMC6546968
- DOI: 10.14814/phy2.14101
Beta-cell excitability and excitability-driven diabetes in adult Zebrafish islets
Abstract
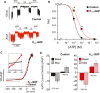
Islet β-cell membrane excitability is a well-established regulator of mammalian insulin secretion, and defects in β-cell excitability are linked to multiple forms of diabetes. Evolutionary conservation of islet excitability in lower organisms is largely unexplored. Here we show that adult zebrafish islet calcium levels rise in response to elevated extracellular [glucose], with similar concentration-response relationship to mammalian β-cells. However, zebrafish islet calcium transients are nor well coupled, with a shallower glucose-dependence of cytoplasmic calcium concentration. We have also generated transgenic zebrafish that conditionally express gain-of-function mutations in ATP-sensitive K+ channels (KATP -GOF) in β-cells. Following induction, these fish become profoundly diabetic, paralleling features of mammalian diabetes resulting from equivalent mutations. KATP -GOF fish become severely hyperglycemic, with slowed growth, and their islets lose glucose-induced calcium responses. These results indicate that, although lacking tight cell-cell coupling of intracellular Ca2+ , adult zebrafish islets recapitulate similar excitability-driven β-cell glucose responsiveness to those in mammals, and exhibit profound susceptibility to diabetes as a result of inexcitability. While illustrating evolutionary conservation of islet excitability in lower vertebrates, these results also provide important validation of zebrafish as a suitable animal model in which to identify modulators of islet excitability and diabetes.
Keywords: KATP; Calcium channels; insulin secretion; metabolism; pancreas; zebrafish.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None.
Figures






References
-
- Antunes, C. M. , Salgado A. P., Rosário L. M., and Santos R. M.. 2000. Differential patterns of glucose‐induced electrical activity and intracellular calcium responses in single mouse and rat pancreatic islets. Diabetes 49:2028–2038. - PubMed
-
- Argenton, F. , Zecchin E., and Bortolussi M.. 1999. Early appearance of pancreatic hormone‐expressing cells in the zebrafish embryo. Mech. Dev. 87:217–221. - PubMed
-
- Bavamian, S. , Klee P., Britan A., Populaire C., Caille D., Cancela J., et al. 2007. Islet‐cell‐to‐cell communication as basis for normal insulin secretion. Diabetes Obes. Metab. 9(Suppl 2):118–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK098584/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 DK 098584/NH/NIH HHS/United States
- R56 DK098584/DK/NIDDK NIH HHS/United States
- DK109407/NH/NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- NIH T32DK108742-01/NH/NIH HHS/United States
- DK 020579/NH/NIH HHS/United States
- R01 DK109407/DK/NIDDK NIH HHS/United States
- T32 DK064466/DK/NIDDK NIH HHS/United States
- T32 DK108742/DK/NIDDK NIH HHS/United States
- R01 DK109407/NH/NIH HHS/United States
- DK98584/NH/NIH HHS/United States
- DK108742/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

