Synaptic Elimination in Neurological Disorders
- PMID: 31161981
- PMCID: PMC7052824
- DOI: 10.2174/1570159X17666190603170511
Synaptic Elimination in Neurological Disorders
Abstract
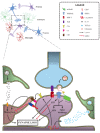
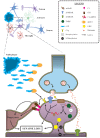
Synapses are well known as the main structures responsible for transmitting information through the release and recognition of neurotransmitters by pre- and post-synaptic neurons. These structures are widely formed and eliminated throughout the whole lifespan via processes termed synaptogenesis and synaptic pruning, respectively. Whilst the first process is needed for ensuring proper connectivity between brain regions and also with the periphery, the second phenomenon is important for their refinement by eliminating weaker and unnecessary synapses and, at the same time, maintaining and favoring the stronger ones, thus ensuring proper synaptic transmission. It is well-known that synaptic elimination is modulated by neuronal activity. However, only recently the role of the classical complement cascade in promoting this phenomenon has been demonstrated. Specifically, microglial cells recognize activated complement component 3 (C3) bound to synapses targeted for elimination, triggering their engulfment. As this is a highly relevant process for adequate neuronal functioning, disruptions or exacerbations in synaptic pruning could lead to severe circuitry alterations that could underlie neuropathological alterations typical of neurological and neuropsychiatric disorders. In this review, we focus on discussing the possible involvement of excessive synaptic elimination in Alzheimer's disease, as it has already been reported dendritic spine loss in post-synaptic neurons, increased association of complement proteins with its synapses and, hence, augmented microglia-mediated pruning in animal models of this disorder. In addition, we briefly discuss how this phenomenon could be related to other neurological disorders, including multiple sclerosis and schizophrenia.
Keywords: Synaptic elimination; alzheimer’s disease; complement cascade; microglia; multiple sclerosis; schizophrenia; synaptic plasticity..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
References
-
- Riccomagno M.M., Kolodkin A.L. Sculpting neural circuits by axon and dendrite pruning. Annu. Rev. Cell Dev. Biol. 2015;31:779–805. [http://dx.doi.org/10.1146/annurev-cellbio-100913-013038]. - PMC - PubMed
-
- Lichtman J.W., Colman H. Synapse elimination and indelible memory. Neuron. 2000;25(2):269–278. [http://dx.doi.org/10.1016/S0896-6273(00)80893-4]. [PMID: 10719884]. - PubMed
-
- Kano M., Hashimoto K. Synapse elimination in the central nervous system. Curr. Opin. Neurobiol. 2009;19(2):154–161. [http://dx.doi.org/10.1016/j.conb.2009.05.002]. [PMID: 19481442]. - PubMed
-
- Yang G., Pan F., Gan W.B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–924. [http://dx.doi.org/10.1038/nature08577]. [PMID: 19946265]. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

