APOLLO-2: A Randomized, Placebo and Active-Controlled Phase III Study Investigating Oliceridine (TRV130), a G Protein-Biased Ligand at the μ-Opioid Receptor, for Management of Moderate to Severe Acute Pain Following Abdominoplasty
- PMID: 31162798
- PMCID: PMC6851842
- DOI: 10.1111/papr.12801
APOLLO-2: A Randomized, Placebo and Active-Controlled Phase III Study Investigating Oliceridine (TRV130), a G Protein-Biased Ligand at the μ-Opioid Receptor, for Management of Moderate to Severe Acute Pain Following Abdominoplasty
Abstract
Objectives: The clinical utility of conventional IV opioids is limited by the occurrence of opioid-related adverse events. Oliceridine is a novel G protein-biased μ-opioid receptor agonist designed to provide analgesia with an improved safety and tolerability profile. This phase III, double-blind, randomized trial (APOLLO-2 [NCT02820324]) evaluated the efficacy and safety of oliceridine for acute pain following abdominoplasty.
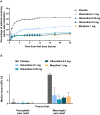
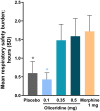
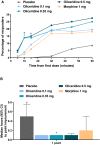
Methods: Patients received a loading dose of either placebo, oliceridine (1.5 mg), or morphine (4 mg), followed by demand doses via patient-controlled analgesia (0.1, 0.35, or 0.5 mg oliceridine; 1 mg morphine; or placebo) with a 6-minute lockout interval. The primary endpoint was the proportion of treatment responders over 24 hours for oliceridine regimens compared to placebo. Secondary outcomes included a predefined composite measure of respiratory safety burden (RSB, representing the cumulative duration of respiratory safety events) and the proportion of treatment responders vs. morphine.
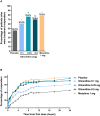
Results: A total of 401 patients were treated with study medication. Effective analgesia was observed for all oliceridine regimens, with responder rates of 61.0%, 76.3%, and 70.0% for the 0.1-, 0.35-, and 0.5-mg regimens, respectively, compared with 45.7% for placebo (all P < 0.05) and 78.3% for morphine. Oliceridine 0.35- and 0.5-mg demand dose regimens were equi-analgesic to morphine using a noninferiority analysis. RSB showed a dose-dependent increase across oliceridine regimens (mean hours [standard deviation], 0.1 mg: 0.43 [1.56]; 0.35 mg: 1.48 [3.83]; 0.5 mg: 1.59 [4.26]; all comparisons not significant at P > 0.05 vs. placebo: 0.60 [2.82]). The RSB measure for morphine was 1.72 (3.86) (P < 0.05 vs. placebo). Gastrointestinal adverse events increased in a dose-dependent manner across oliceridine demand dose regimens (0.1 mg: 49.4%; 0.35 mg: 65.8%; 0.5 mg: 78.8%; vs. placebo: 47.0%; and morphine: 79.3%). In comparison to morphine, the proportion of patients experiencing nausea or vomiting was lower with the 2 equi-analgesic dose regimens of 0.35 and 0.5 mg oliceridine.
Conclusions: Oliceridine is a safe and effective IV analgesic for the relief of moderate to severe acute postoperative pain in patients undergoing abdominoplasty. Since the low-dose regimen of 0.1 mg oliceridine was superior to placebo but not as effective as the morphine regimen, safety comparisons to morphine are relevant only to the 2 equi-analgesic dose groups of 0.35 and 0.5 mg, which showed a favorable safety and tolerability profile regarding respiratory and gastrointestinal adverse effects compared to morphine. These findings support that oliceridine may provide a new treatment option for patients with moderate to severe acute pain where an IV opioid is warranted.
Keywords: abdominoplasty; analgesia; clinical trial; patient controlled; postoperative.
© 2019 The Authors. Pain Practice published by Wiley Periodicals, Inc. on behalf of World Institute of Pain.
Conflict of interest statement
Mark Demitrack is a full‐time employees of Trevena Inc. and owns stock in Trevena Inc. David G. Soergel, Franck Skobieranda, Monica Salamea and David A. Burt were full‐time employees, and stockholders, of Trevena Inc. at the time the research was conducted and the manuscript was fully written. Neil Singla is the founder and CEO of Lotus Clinical Research LLC, an analgesic CRO and research site; in this capacity he has served as a consultant and provided clinical trial services for Trevena Inc. Eugene R. Viscusi has served as a consultant to Trevena Inc.
Figures



References
-
- Thomazeau J, Rouquette A, Martinez V, et al. Acute pain factors predictive of post‐operative pain and opioid requirement in multimodal analgesia following knee replacement. Eur J Pain. 2016;20:822–832. - PubMed
-
- Fiala T. Tranversus abdominis plane block during abdominoplasty to improve postoperative patient comfort. Aesthet Surg J. 2015;35:72–80. - PubMed
-
- Morales R Jr, Mentz H 3rd, Newall G, Patronella C, Masters O 3rd. Use of abdominal field block injections with liposomal bupivicaine to control postoperative pain after abdominoplasty. Aesthet Surg J. 2013;33:1148–1153. - PubMed
-
- Feng LJ. Painless abdominoplasty: the efficacy of combined intercostal and pararectus blocks in reducing postoperative pain and recovery time. Plast Reconstr Surg. 2010;126:1723–1732. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

