The prediction of early preeclampsia: Results from a longitudinal proteomics study
- PMID: 31163045
- PMCID: PMC6548389
- DOI: 10.1371/journal.pone.0217273
The prediction of early preeclampsia: Results from a longitudinal proteomics study
Abstract
Objectives: To identify maternal plasma protein markers for early preeclampsia (delivery <34 weeks of gestation) and to determine whether the prediction performance is affected by disease severity and presence of placental lesions consistent with maternal vascular malperfusion (MVM) among cases.
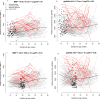
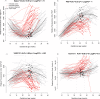
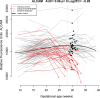
Study design: This longitudinal case-control study included 90 patients with a normal pregnancy and 33 patients with early preeclampsia. Two to six maternal plasma samples were collected throughout gestation from each woman. The abundance of 1,125 proteins was measured using high-affinity aptamer-based proteomic assays, and data were modeled using linear mixed-effects models. After data transformation into multiples of the mean values for gestational age, parsimonious linear discriminant analysis risk models were fit for each gestational-age interval (8-16, 16.1-22, 22.1-28, 28.1-32 weeks). Proteomic profiles of early preeclampsia cases were also compared to those of a combined set of controls and late preeclampsia cases (n = 76) reported previously. Prediction performance was estimated via bootstrap.
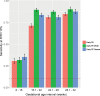
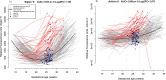
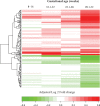
Results: We found that 1) multi-protein models at 16.1-22 weeks of gestation predicted early preeclampsia with a sensitivity of 71% at a false-positive rate (FPR) of 10%. High abundance of matrix metalloproteinase-7 and glycoprotein IIbIIIa complex were the most reliable predictors at this gestational age; 2) at 22.1-28 weeks of gestation, lower abundance of placental growth factor (PlGF) and vascular endothelial growth factor A, isoform 121 (VEGF-121), as well as elevated sialic acid binding immunoglobulin-like lectin 6 (siglec-6) and activin-A, were the best predictors of the subsequent development of early preeclampsia (81% sensitivity, FPR = 10%); 3) at 28.1-32 weeks of gestation, the sensitivity of multi-protein models was 85% (FPR = 10%) with the best predictors being activated leukocyte cell adhesion molecule, siglec-6, and VEGF-121; 4) the increase in siglec-6, activin-A, and VEGF-121 at 22.1-28 weeks of gestation differentiated women who subsequently developed early preeclampsia from those who had a normal pregnancy or developed late preeclampsia (sensitivity 77%, FPR = 10%); 5) the sensitivity of risk models was higher for early preeclampsia with placental MVM lesions than for the entire early preeclampsia group (90% versus 71% at 16.1-22 weeks; 87% versus 81% at 22.1-28 weeks; and 90% versus 85% at 28.1-32 weeks, all FPR = 10%); and 6) the sensitivity of prediction models was higher for severe early preeclampsia than for the entire early preeclampsia group (84% versus 71% at 16.1-22 weeks).
Conclusion: We have presented herein a catalogue of proteome changes in maternal plasma proteome that precede the diagnosis of preeclampsia and can distinguish among early and late phenotypes. The sensitivity of maternal plasma protein models for early preeclampsia is higher in women with underlying vascular placental disease and in those with a severe phenotype.
Conflict of interest statement
The authors have declared that no competing interests exist. ALT, TC, SSH, and RR are co-authors of an invention disclosure based on results from this study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Romero R (1996) The child is the father of the man. Prenat Neonat Med 1: 8–11.
-
- Romero R, Lockwood C, Oyarzun E, Hobbins JC (1988) Toxemia: new concepts in an old disease. Semin Perinatol 12: 302–323. - PubMed
-
- Vatten LJ, Skjaerven R (2004) Is pre-eclampsia more than one disease? Bjog 111: 298–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

