Route of antibiotic prophylaxis for prevention of cerebrospinal fluid-shunt infection
- PMID: 31163089
- PMCID: PMC6548496
- DOI: 10.1002/14651858.CD012902.pub2
Route of antibiotic prophylaxis for prevention of cerebrospinal fluid-shunt infection
Abstract
Background: The main complication of cerebrospinal fluid (CSF) shunt surgery is shunt infection. Prevention of these shunt infections consists of the perioperative use of antibiotics that can be administered in five different ways: orally; intravenously; intrathecally; topically; and via the implantation of antibiotic-impregnated shunt catheters.
Objectives: To determine the effect of different routes of antibiotic prophylaxis (i.e. oral, intravenous, intrathecal, topical and via antibiotic-impregnated shunt catheters) on CSF-shunt infections in persons treated for hydrocephalus using internalised CSF shunts.
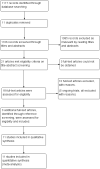
Search methods: We conducted a systematic electronic search without restrictions on language, date or publication type. We performed the search on the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE and Embase, with the help of the Information Specialist of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group. The search was performed in January 2018.
Selection criteria: All randomised and quasi-randomised controlled trials that studied the effect of antibiotic prophylaxis, in any dose or administration route, for the prevention of CSF-shunt infection in patients that were treated with an internal cerebrospinal fluid shunt. Patients with external shunts were not eligible.
Data collection and analysis: Two review authors independently extracted data from included studies. We resolved disagreements by discussion or by referral to an independent researcher within our department when necessary. Analyses were also performed by at least two authors.
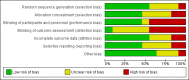
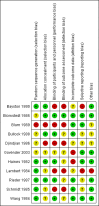
Main results: We included a total of 11 small randomised controlled trials, containing 1109 participants, in this systematic review. Three of these studies included solely children, and the remaining eight included participants of all ages. Most studies were limited to the evaluation of ventriculoperitoneal shunts. However, five studies included participants with ventriculoatrial shunts, of which one study contained four participants with a subduroperitoneal shunt. We judged four out of 11 (36%) trials at unclear risk of bias, while the remaining seven trials (64%) scored high risk of bias in one or more of the components assessed.We analysed all included studies in order to estimate the effect of antibiotic prophylaxis on the proportion of shunt infections regardless of administration route. Although the quality of evidence in these studies was low, there may be a positive effect of antibiotic prophylaxis on the number of participants who had shunt infections (RR 0.55, 95% CI 0.36 to 0.84), meaning a 55% reduction in the number of participants who had shunt infection compared with standard care or placebo.Within the different administration routes, only within intravenous administration of antibiotic prophylaxis there may be evidence of an effect on the risk of shunt infections (RR 0.55, 95% CI 0.33 to 0.90). However, this was the only route that contained more than two studies (8 studies; 797 participants). Evidence was uncertain for both, intrathecal administration of antibiotics (RR 0.73, 95% CI 0.28 to 1.93, 2 studies; 797 participants; low quality evidence) and antibiotic impregnated catheters (RR 0.36, 95% CI 0.10 to 1.24, 1 study; 110 participants; very low quality evidence) AUTHORS' CONCLUSIONS: Antibiotic prophylaxis may have a positive effect on lowering the number of participants who had shunt infections. However, the quality of included studies was low and the effect is not consistent within the different routes of administration that have been analysed. It is therefore uncertain whether prevention of shunt infection varies by different antibiotic agents, different administration routes, timing and doses; or by characteristics of patients, e.g. children and adults. The results of the review should be seen as hypothesis-generating rather than definitive, and the results should be confirmed in adequately powered trials or large multicentre studies in order to obtain high-quality evidence in the field of ventricular shunt infection prevention.
Conflict of interest statement
SA ‒ none
HB ‒ none
EvL ‒ none
Figures






Update of
References
References to studies included in this review
Bayston 1990 {published data only}
-
- Bayston R, Bannister C, Boston V, Burman R, Burns B, Cooke F, et al. A prospective randomised controlled trial of antimicrobial prophylaxis in hydrocephalus shunt surgery. Zeitschrift fur Kinderchirurgie [Surgery in Infancy and Childhood] 1991;45(Suppl 1):5‐7. - PubMed
Blomstedt 1985 {published data only}
-
- Blomstedt GC. Results of trimethoprim‐sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures: A double‐blind randomized trial. Journal of Neurosurgery 1985;62(5):694‐7. - PubMed
Blum 1989 {published data only}
-
- Blum J, Schwarz M, Voth D. Antibiotic single‐dose prophylaxis of shunt infections. Neurosurgical Review 1989;12(3):239‐44. - PubMed
Bullock 1988 {published data only}
-
- Bullock R, Dellen JR, Ketelbey W, Reinach SG. A double‐blind placebo‐controlled trial of perioperative prophylactic antibiotics for elective neurosurgery. Journal of Neurosurgery 1988;69(5):687‐91. - PubMed
Djindjian 1986 {published data only}
-
- Djindjian M, Fevrier M, Otterbein G, Soussy J. Oxacillin prophylaxis in cerebrospinal fluid shunt procedures: results of a randomized open study in 60 hydrocephalic patients. Surgical Neurology 1986;25(2):178‐80. - PubMed
Govender 2003 {published data only}
-
- Govender ST, Nathoo N, Dellen JR. Evaluation of an antibiotic‐impregnated shunt system for the treatment of hydrocephalus. Journal of Neurosurgery 2003;99(5):831‐9. - PubMed
Haines 1982 {published data only}
-
- Haines SJ, Taylor F. Prophylactic methicillin for shunt operations: effects on incidence of shunt malfunction and infection. Child's Brain 1982;9(1):10‐22. - PubMed
Lambert 1984 {published data only}
-
- Lambert M, MacKinnon AE, Vaishnav A. Comparison of two methods of prophylaxis against CSF shunt infection. Zeitschrift fur Kinderchirurgie [Surgery in Infancy and Childhood] 1984;39(Suppl 2):109‐10. - PubMed
Rieder 1987 {published data only}
Schmidt 1985 {published data only}
-
- Schmidt K, Gjerris F, Osgaard O, Hvidberg EF, Kristiansen JE, Dahlerup B, et al. Antibiotic prophylaxis in cerebrospinal fluid shunting: a prospective randomized trial in 152 hydrocephalic patients. Neurosurgery 1985;17(1):1‐5. - PubMed
Wang 1984 {published data only}
-
- Wang EE, Prober CG, Hendrick BE, Hoffman HJ, Humphreys RP. Prophylactic sulfamethoxazole and trimethoprim in ventriculoperitoneal shunt surgery A double‐blind, randomized, placebo‐controlled trial. JAMA 1984;251(9):1174‐7. - PubMed
References to studies excluded from this review
Eymann 2008 {published data only}
-
- Eymann R, Chehab S, Strowitzki M, Steudel W, Kiefer M. Clinical and economic consequences of antibiotic‐impregnated cerebrospinal fluid shunt catheters. Journal of Neurosurgery. Pediatrics 2008;1(6):444‐50. - PubMed
ISRCTN29451493 {published data only}
-
- ISRCTN29451493. Silver impregnated line versus external ventricular drains (EVD) randomised trial [Silver impregnated line versus EVD randomised trial and cerebrospinal fluid infection from the use of external ventricular drains]. www.isrctn.com/ISRCTN29451493 (first received 12 September 2015).
Kumar 2016 {published data only}
-
- Kumar R, Younus SM, Haseeb A, Bilal M, Zeeshan QM, Ashraf J. Frequency of infection associated with ventriculo‐peritoneal shunt placement. Journal of the Pakistan Medical Association 2016;66(7):815‐8. - PubMed
Moussa 2017 {published data only}
-
- Moussa WM, Mohamed MA. Efficacy of postoperative antibiotic injection in and around ventriculoperitoneal shunt in reduction of shunt infection a randomized controlled trial. Clinical Neurology and Neurosurgery 2017;143:144‐9. - PubMed
NCT00280904 {published data only}
-
- NCT00280904. A registry for comparing catheter‐related infection rates among various shunt systems in the treatment of hydrocephalus [A Registry for Comparing Catheter‐Related Infection Rates Among Various Shunt Systems in the Treatment of Hydrocephalus]. clinicaltrials.gov/ct2/show/NCT00280904 (first received 24 January 2006).
NCT00286104 {published data only}
-
- NCT00286104. Impact of ventricular catheter used with antimicrobial agents on patients with a ventricular catheter [The impact of ventricular catheter impregnated with antimicrobial agents on infection in patients with ventricular catheter: a prospective randomized study]. clinicaltrials.gov/ct2/show/NCT00286104 (first received 3 February 2006).
NCT01936272 {published data only}
-
- NCT01936272. Randomized controlled trial of shunt vs ETV/CPC for PIH in Ugandan infants [Neurocognitive outcomes and changes in brain and cerebral spinal fluid (CSF) volume after treatment of post‐infectious hydrocephalus (PIH) in Ugandan infants by shunting versus ETV/CPC]. clinicaltrials.gov/ct2/show/NCT01936272 (first received 6 September 2013).
NCT02600793 {published data only}
-
- NCT02600793. Ceftaroline diffusion into cerebrospinal fluid of children [Ceftaroline diffusion into cerebrospinal fluid of children with ventriculitis due to ventriculoperitoneal shunt (VPS) infection]. clinicaltrials.gov/ct2/show/NCT02600793 (first received 9 November 2015).
Nejat 2008 {published data only}
-
- Nejat F, Tajik P, Khashab M, Kazmi SS, Khotaei GT, Salahesh S. A randomized trial of ceftriaxone versus trimethoprim‐sulfamethoxazole to prevent ventriculoperitoneal shunt infection. Journal of Microbiology, Immunology and Infection 2008;41(2):112‐7. - PubMed
Odio 1984 {published data only}
-
- Odio C, Mohs E, Sklar FH, Nelson JD, McCracken GH. Adverse reactions to vancomycin used as prophylaxis for CSF shunt procedures. American Journal of Diseases of Children (1960) 1984;138(1):17‐9. - PubMed
Tacconelli 2008 {published data only}
-
- Tacconelli E, Cataldo MA, Albanese A, Tumbarello M, Arduini E, Spanu T, et al. Vancomycin versus cefazolin prophylaxis for cerebrospinal shunt placement in a hospital with a high prevalence of meticillin‐resistant Staphylococcus aureus. Journal of Hospital Infection 2008;69(4):337‐44. - PubMed
Theophilus 2011 {published data only}
Walters 1992 {published data only}
-
- Walters BC, Goumnerova L, Hoffman HJ, Hendrick EB, Humphreys RP, Levinton C. A randomized controlled trial of perioperative rifampin/trimethoprim in cerebrospinal fluid shunt surgery. Child's Nervous System : ChNS : Official Journal of the International Society for Pediatric Neurosurgery 1992;8(5):253‐7. - PubMed
Young 1987 {published data only}
-
- Young RF, Lawner PM. Perioperative antibiotic prophylaxis for prevention of postoperative neurosurgical infections. A randomized clinical trial. Journal of Neurosurgery 1987;66(5):701‐5. - PubMed
Zentner 1995 {published data only}
-
- Zentner J, Gilsbach J, Felder T. Antibiotic prophylaxis in cerebrospinal fluid shunting: a prospective randomized trial in 129 patients. Neurosurgical Review 1995;18(3):169‐72. - PubMed
Additional references
Attenello 2010
-
- Attenello FJ, Garces‐Ambrossi GL, Zaidi HA, Sciubba DM, Jallo GI. Hospital costs associated with shunt infections in patients receiving antibiotic‐impregnated shunt catheters versus standard shunt catheters. Neurosurgery 2010;66(2):284‐9. - PubMed
Bondurant 1995
-
- Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatric Neurosurgery 1995;23(5):254‐8. - PubMed
Borgbjerg 1995
-
- Borgbjerg BM, Gjerris F, Albeck MJ, Borgesen SE. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first‐time shunts. Acta Neurochirurgica 1995;136(1‐2):1‐7. - PubMed
Casey 1997
-
- Casey AT, Kimmings EJ, Kleinlugtebeld AD, Taylor WA, Harkness WF, Hayward RD. The long‐term outlook for hydrocephalus in childhood. A ten‐year cohort study of 155 patients. Pediatric Neurosurgery 1997;27(2):63‐70. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Drake 1998
-
- Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 1998;43(2):294‐303. - PubMed
Frame 1984
-
- Frame PT, McLaurin RL. Treatment of CSF shunt infections with intrashunt plus oral antibiotic therapy. Journal of Neurosurgery 1984;60(2):354‐60. - PubMed
Fu 2002
-
- Fu L, Tang Y, Wang S. Effect of nonoperative treatment on the outcome of patients with posttraumatic hydrocephalus. Chinese Journal of Traumatology 2002;5(1):7‐11. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version 3.6. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greenberg 2010
-
- Greenberg MS. Shunt infection. Handbook of Neurosurgery. 7th Edition. New York: Thieme, 2010:345‐8.
Guyatt 2008
Hebb 2001
-
- Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 2001;49(5):1166‐84. - PubMed
Higgins 2011a
-
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Green S, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
James 2014
-
- James G, Hartley JC, Morgan RD, Ternier J. Effect of introduction of antibiotic‐impregnated shunt catheters on cerebrospinal fluid shunt infection in children: a large single‐center retrospective study. Journal of Neurosurgery. Pediatrics 2014;13(1):101‐6. - PubMed
Kestle 2011
-
- Kestle JR, Riva‐Cambrin J, Wellons JC 3rd, Kulkarni AV, Whitehead WE, Walker ML, et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. Journal of Neurosurgery. Pediatrics 2011;8(1):22‐9. - PMC - PubMed
Kestle 2016
-
- Kestle JR, Holubkov R, Cochrane D, Kulkarni AV, Limbrick DD, Luerssen TG, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. Journal of Neurosurgery. Pediatrics 2016;17(4):391‐6. - PubMed
Klimo 2014
-
- Klimo P, Poppel M, Thompson CJ, Baird LC, Duhaime AC, Flannery AM, et al. Pediatric hydrocephalus: systematic literature review and evidence‐based guidelines. Part 6: Preoperative antibiotics for shunt surgery in children with hydrocephalus: a systematic review and meta‐analysis. Journal of Neurosurgery. Pediatrics 2014;14(Suppl 1):44‐52. - PubMed
Konstantelias 2015
-
- Konstantelias AA, Vardakas KZ, Polyzos KA, Tansarli GS, Falagas ME. Antimicrobial‐impregnated and ‐coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta‐analysis. Journal of Neurosurgery 2015;122(5):1096‐112. - PubMed
Lefevbre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Limbrick 2014
-
- Limbrick DD, Baird LC, Klimo P, Riva‐Cambrin J, Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence‐based guidelines. Part 4: Cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. Journal of Neurosurgery. Pediatrics 2014;14(Suppl 1):30‐4. - PubMed
Moussa 2016
-
- Moussa WM, Mohamed MA. Efficacy of postoperative antibiotic injection in and around ventriculoperitoneal shunt in reduction of shunt infection: A randomized controlled trial. Clinical Neurology and Neurosurgery 2016;143:144‐9. - PubMed
Ragel 2006
-
- Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. Journal of Neurosurgery 2006;105(2):242‐7. - PubMed
Ratilal 2008
-
- Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. Journal of Neurosurgery. Pediatrics 2008;1(1):48‐56. - PubMed
Rekate 1999
-
- Rekate HL. Treatment of hydrocephalus. In: Albright AL, Pollack IF, Adelson PD editor(s). Principles and Practice of Pediatric Neurosurgery. New York: Thieme, 1999:47‐75.
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sciubba 2007
-
- Sciubba DM, Lin LM, Woodworth GF, McGirt MJ, Carson B, Jallo GI. Factors contributing to the medical costs of cerebrospinal fluid shunt infection treatment in pediatric patients with standard shunt components compared with those in patients with antibiotic impregnated components. Neurosurgical Focus 2007;22(4):E9. - PubMed
Simon 2009
Simon 2010
van Lindert 2018
Yogev 1985
-
- Yogev R. Cerebrospinal fluid shunt infections: a personal view. Pediatric Infectious Disease 1985;4(2):113‐8. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

