Emerging Function and Clinical Values of Exosomal MicroRNAs in Cancer
- PMID: 31163321
- PMCID: PMC6545365
- DOI: 10.1016/j.omtn.2019.04.027
Emerging Function and Clinical Values of Exosomal MicroRNAs in Cancer
Abstract
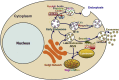
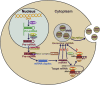
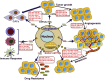
Exosomes are a subset of membrane-bound extracellular vesicles with diameters ranging from 30 to 100 nm. Exosomes enclose a variety of molecules, such as lipids, proteins, and non-coding RNAs. In the past decades, microRNAs (miRNAs) have attracted great attention in cancer research, as they play an important role in the occurrence and development of cancer. Increasing evidence indicates that tumor cells communicate with not only other tumor cells but also cells present in the tumor microenvironment via secretion and transfer of exosomal miRNAs. More importantly, exosomal miRNAs are found to serve as signaling molecules to regulate tumor growth, angiogenesis, metastasis, sensitivity to chemotherapy, and immune evasion. Deregulated expression of exosomal miRNAs is an early event in carcinogenesis and may reflect the malignant characteristics of cancer. Owing to the wide existence and high stability of exosomal miRNAs in body fluids, they may represent a novel class of non-invasive biomarkers for cancer. In this review, we highlight the recent advances on the functional role of exosomal miRNAs in cancer pathogenesis. We also discuss the potential clinical utility of exosome-shuttled miRNAs as biomarkers for the diagnosis and treatment of cancer.
Keywords: cancer biomarker; cancer diagnosis; cancer pathogenesis; cancer treatment; carcinogenesis; exosome; microRNA.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Abak A., Abhari A., Rahimzadeh S. Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PeerJ. 2018;6:e4763. - PMC - PubMed
- Abak, A., Abhari, A., and Rahimzadeh, S. (2018). Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PeerJ 6, e4763. - PMC - PubMed
-
- van Niel G., Raposo G., Candalh C., Boussac M., Hershberg R., Cerf-Bensussan N., Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. - PubMed
- van Niel, G., Raposo, G., Candalh, C., Boussac, M., Hershberg, R., Cerf-Bensussan, N., and Heyman, M. (2001). Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121, 337-349. - PubMed
-
- Blanchard N., Lankar D., Faure F., Regnault A., Dumont C., Raposo G., Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 2002;168:3235–3241. - PubMed
- Blanchard, N., Lankar, D., Faure, F., Regnault, A., Dumont, C., Raposo, G., and Hivroz, C. (2002). TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 168, 3235-3241. - PubMed
-
- Kharaziha P., Ceder S., Li Q., Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim. Biophys. Acta. 2012;1826:103–111. - PubMed
- Kharaziha, P., Ceder, S., Li, Q., and Panaretakis, T. (2012). Tumor cell-derived exosomes: a message in a bottle. Biochim. Biophys. Acta 1826, 103-111. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

