A Focus on the Functions of Area 25
- PMID: 31163643
- PMCID: PMC6627335
- DOI: 10.3390/brainsci9060129
A Focus on the Functions of Area 25
Abstract
Subcallosal area 25 is one of the least understood regions of the anterior cingulate cortex, but activity in this area is emerging as a crucial correlate of mood and affective disorder symptomatology. The cortical and subcortical connectivity of area 25 suggests it may act as an interface between the bioregulatory and emotional states that are aberrant in disorders such as depression. However, evidence for such a role is limited because of uncertainty over the functional homologue of area 25 in rodents, which hinders cross-species translation. This emphasizes the need for causal manipulations in monkeys in which area 25, and the prefrontal and cingulate regions in which it is embedded, resemble those of humans more than rodents. In this review, we consider physiological and behavioral evidence from non-pathological and pathological studies in humans and from manipulations of area 25 in monkeys and its putative homologue, the infralimbic cortex (IL), in rodents. We highlight the similarities between area 25 function in monkeys and IL function in rodents with respect to the regulation of reward-driven responses, but also the apparent inconsistencies in the regulation of threat responses, not only between the rodent and monkey literatures, but also within the rodent literature. Overall, we provide evidence for a causal role of area 25 in both the enhanced negative affect and decreased positive affect that is characteristic of affective disorders, and the cardiovascular and endocrine perturbations that accompany these mood changes. We end with a brief consideration of how future studies should be tailored to best translate these findings into the clinic.
Keywords: anhedonia; anticipatory arousal; area 25; autonomic; emotion; infralimbic; negative affect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
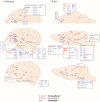
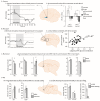

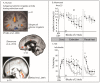
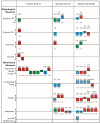
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

