BioPATH: A Biomarker Study in Asian Patients with HER2+ Advanced Breast Cancer Treated with Lapatinib and Other Anti-HER2 Therapy
- PMID: 31163957
- PMCID: PMC6790855
- DOI: 10.4143/crt.2018.598
BioPATH: A Biomarker Study in Asian Patients with HER2+ Advanced Breast Cancer Treated with Lapatinib and Other Anti-HER2 Therapy
Abstract
Purpose: BioPATH is a non-interventional study evaluating the relationship of molecular biomarkers (PTEN deletion/downregulation, PIK3CA mutation, truncated HER2 receptor [p95HER2], and tumor HER2 mRNA levels) to treatment responses in Asian patients with HER2+ advanced breast cancer treated with lapatinib and other HER2-targeted agents.
Materials and methods: Female Asian HER2+ breast cancer patients (n=154) who were candidates for lapatinib-based treatment following metastasis and having an available primary tumor biopsy specimen were included. The primary endpoint was progression-free survival (PFS). Secondary endpoints were response rate, overall survival on lapatinib, correlation between biomarker status and PFS for any previous trastuzumab-based treatment, and conversion/conservation rates of the biomarker status between tissue samples collected at primary diagnosis and at recurrence/metastasis. Potential relationships between tumor mRNA levels of HER2 and response to lapatinib-based therapy were also explored.
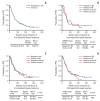
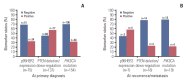
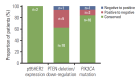
Results: p95HER2, PTEN deletion/downregulation, and PIK3CA mutation did not demonstrate any significant co-occurrence pattern and were not predictive of clinical outcomes on either lapatinib-based treatment or any previous trastuzumab-based therapy in the metastatic setting. Proportions of tumors positive for p95HER2 expression, PIK3CA mutation, and PTEN deletion/down-regulation at primary diagnosis were 32%, 31.2%, and 56.2%, respectively. Despite limited availability of paired samples, biomarker status patterns were conserved in most samples. HER2 mRNA levels were not predictive of PFS on lapatinib.
Conclusion: The prevalence of p95HER2 expression, PIK3CA mutation, and PTEN deletion/downregulation at primary diagnosis were similar to previous reports. Importantly, no difference was observed in clinical outcome based on the status of these biomarkers, consistent with reports from other studies.
Keywords: Biomarkers; Breast neoplasms; HER2; Lapatinib; Trastuzumab.
Conflict of interest statement
Sung-Bae Kim has received funding from Novartis, Sanofi Aventis, Kyowa Kirim Inc., Dongkook Pharmaceuticals Company Ltd. Gerardo Cornelio has received funding from Renovo; has served as a consultant/advisory role for Astra Zeneca Oncology, BF, MSD Oncology, and Esai Oncology. Yoon Sim Yap has served as a consultant/advisory role from Novartis. Soonmyung Paik has received funding from GSK for this study; has served as a consultant/advisory role for Leica Biosystems. Christos Nathaniel is an employee of Novartis Pharmaceuticals Corporation. The following authors have nothing to disclose: In-Gu Do, Janice Tsang, Tae-You Kim, Gyungyub Gong, Suee Lee, Ting-Ying Ng, Sarah Park, Ho-Suk Oh, Joanne Chiu, Joohyuk Sohn, Moonhee Lee, Young-Jin Choi, Eun Mi Lee, Kyong-Hwa Park, and Jungsil Ro.
Figures

Similar articles
-
Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer.Breast Cancer Res Treat. 2018 Feb;167(3):731-740. doi: 10.1007/s10549-017-4533-9. Epub 2017 Nov 7. Breast Cancer Res Treat. 2018. PMID: 29110152 Free PMC article. Clinical Trial.
-
Role of HER2-Related Biomarkers (HER2, p95HER2, HER3, PTEN, and PIK3CA) in the Efficacy of Lapatinib plus Capecitabine in HER2-Positive Advanced Breast Cancer Refractory to Trastuzumab.Oncology. 2017;93(1):51-61. doi: 10.1159/000468521. Epub 2017 May 6. Oncology. 2017. PMID: 28478451
-
Impact of somatic PI3K pathway and ERBB family mutations on pathological complete response (pCR) in HER2-positive breast cancer patients who received neoadjuvant HER2-targeted therapies.Breast Cancer Res. 2017 Jul 27;19(1):87. doi: 10.1186/s13058-017-0883-9. Breast Cancer Res. 2017. PMID: 28750640 Free PMC article. Clinical Trial.
-
Lapatinib in breast cancer - the predictive significance of HER1 (EGFR), HER2, PTEN and PIK3CA genes and lapatinib plasma level assessment.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010 Dec;154(4):281-8. doi: 10.5507/bp.2010.043. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010. PMID: 21293538 Review.
-
Lapatinib.Recent Results Cancer Res. 2018;211:19-44. doi: 10.1007/978-3-319-91442-8_2. Recent Results Cancer Res. 2018. PMID: 30069757 Review.
Cited by
-
Comparison of PIK3CA Mutation Prevalence in Breast Cancer Across Predicted Ancestry Populations.JCO Precis Oncol. 2022 Nov;6:e2200341. doi: 10.1200/PO.22.00341. JCO Precis Oncol. 2022. PMID: 36446041 Free PMC article.
-
PIK3CA Mutation is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients.Cancer Res Treat. 2023 Apr;55(2):531-541. doi: 10.4143/crt.2022.221. Epub 2022 Sep 7. Cancer Res Treat. 2023. PMID: 36097803 Free PMC article.
-
Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments.Med Sci (Basel). 2020 Mar 23;8(1):18. doi: 10.3390/medsci8010018. Med Sci (Basel). 2020. PMID: 32210163 Free PMC article. Review.
-
Discordance of PIK3CA mutational status between primary and metastatic breast cancer: a systematic review and meta-analysis.Breast Cancer Res Treat. 2023 Sep;201(2):161-169. doi: 10.1007/s10549-023-07010-1. Epub 2023 Jul 1. Breast Cancer Res Treat. 2023. PMID: 37392328 Free PMC article.
References
-
- Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–85. - PubMed
-
- Katanoda K, Matsuda T, Matsuda A, Shibata A, Nishino Y, Fujita M, et al. An updated report of the trends in cancer incidence and mortality in Japan. Jpn J Clin Oncol. 2013;43:492–507. - PubMed
-
- Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

