Rationale and design of OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people (OPERAM): a cluster randomised controlled trial
- PMID: 31164366
- PMCID: PMC6561415
- DOI: 10.1136/bmjopen-2018-026769
Rationale and design of OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people (OPERAM): a cluster randomised controlled trial
Abstract
Introduction: Multimorbidity and polypharmacy are important risk factors for drug-related hospital admissions (DRAs). DRAs are often linked to prescribing problems (overprescribing and underprescribing), as well as non-adherence with drug regimens for different reasons. In this trial, we aim to assess whether a structured medication review compared with standard care can reduce DRAs in multimorbid older patients with polypharmacy.
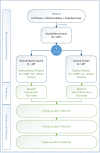
Methods and analysis: OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people is a European multicentre, cluster randomised, controlled trial. Hospitalised patients ≥70 years with ≥3 chronic medical conditions and concurrent use of ≥5 chronic medications are included in the four participating study centres of Bern (Switzerland), Utrecht (The Netherlands), Brussels (Belgium) and Cork (Ireland). Patients treated by the same prescribing physician constitute a cluster, and clusters are randomised 1:1 to either standard care or Systematic Tool to Reduce Inappropriate Prescribing (STRIP) intervention with the help of a clinical decision support system, the STRIP Assistant. STRIP is a structured method performing customised medication reviews, based on Screening Tool of Older People's Prescriptions/Screening Tool to Alert to Right Treatment criteria to detect potentially inappropriate prescribing. The primary endpoint is any DRA where the main reason or a contributory reason for the patient's admission is caused by overtreatment or undertreatment, and/or inappropriate treatment. Secondary endpoints include number of any hospitalisations, all-cause mortality, number of falls, quality of life, degree of polypharmacy, activities of daily living, patient's drug compliance, the number of significant drug-drug interactions, drug overuse and underuse and potentially inappropriate medication.
Ethics and dissemination: The local Ethics Committees in Switzerland, Ireland, The Netherlands and Belgium approved this trial protocol. We will publish the results of this trial in a peer-reviewed journal.
Main funding: European Union's Horizon 2020 programme.
Trial registration number: NCT02986425 , SNCTP000002183 , NTR6012, U1111-1181-9400.
Keywords: clinical pharmacology; general medicine (see internal medicine); geriatric medicine; internal medicine.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Menotti A, Mulder I, Nissinen A, et al. . Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: The FINE study (Finland, Italy, Netherlands, Elderly). J Clin Epidemiol 2001;54:680–6. - PubMed
-
- A G. Making the case for ongoing care. 2010. http://www.rwjf.org/en/research-publications/find-rwjf-research/2010/02/....