Indole Signaling at the Host-Microbiota-Pathogen Interface
- PMID: 31164470
- PMCID: PMC6550529
- DOI: 10.1128/mBio.01031-19
Indole Signaling at the Host-Microbiota-Pathogen Interface
Erratum in
-
Erratum for Kumar et al., "Indole Signaling at the Host-Microbiota-Pathogen Interface".mBio. 2020 Feb 4;11(1):e03318-19. doi: 10.1128/mBio.03318-19. mBio. 2020. PMID: 32019796 Free PMC article. No abstract available.
Abstract
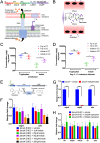
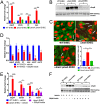
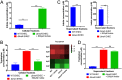
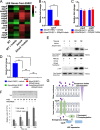
Microbial establishment within the gastrointestinal (GI) tract requires surveillance of the gut biogeography. The gut microbiota coordinates behaviors by sensing host- or microbiota-derived signals. Here we show for the first time that microbiota-derived indole is highly prevalent in the lumen compared to the intestinal tissue. This difference in indole concentration plays a key role in modulating virulence gene expression of the enteric pathogens enterohemorrhagic Escherichia coli (EHEC) and Citrobacter rodentium Indole decreases expression of genes within the locus of enterocyte effacement (LEE) pathogenicity island, which is essential for these pathogens to form attaching and effacing (AE) lesions on enterocytes. We synthetically altered the concentration of indole in the GI tracts of mice by employing mice treated with antibiotics to deplete the microbiota and reconstituted with indole-producing commensal Bacteroides thetaiotaomicron (B. theta) or a B. theta ΔtnaA mutant (does not produce indole) or by engineering an indole-producing C. rodentium strain. This allowed us to assess the role of self-produced versus microbiota-produced indole, and the results show that decreased indole concentrations promote bacterial pathogenesis, while increased levels of indole decrease bacterial virulence gene expression. Moreover, we identified the bacterial membrane-bound histidine sensor kinase (HK) CpxA as an indole sensor. Enteric pathogens sense a gradient of indole concentrations in the gut to probe different niches and successfully establish an infection.IMPORTANCE Pathogens sense and respond to several small molecules within the GI tract to modulate expression of their virulence repertoire. Indole is a signaling molecule produced by the gut microbiota. Here we show that indole concentrations are higher in the lumen, where the microbiota is present, than in the intestinal tissue. The enteric pathogens EHEC and C. rodentium sense indole to downregulate expression of their virulence genes, as a read-out of the luminal compartment. We also identified the bacterial membrane-bound HK CpxA as an indole sensor. This regulation ensures that EHEC and C. rodentium express their virulence genes only at the epithelial lining, which is the niche they colonize.
Keywords: Citrobacter rodentium; CpxA; enterohemorrhagic E. coli (EHEC); indole; locus of enterocyte effacement (LEE); microbiota.
Copyright © 2019 Kumar and Sperandio.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
