A User's Guide to Cell-Free Protein Synthesis
- PMID: 31164605
- PMCID: PMC6481089
- DOI: 10.3390/mps2010024
A User's Guide to Cell-Free Protein Synthesis
Abstract
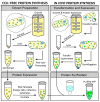
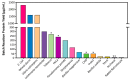
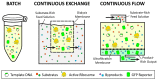
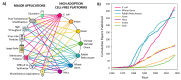
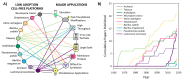
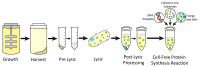
Cell-free protein synthesis (CFPS) is a platform technology that provides new opportunities for protein expression, metabolic engineering, therapeutic development, education, and more. The advantages of CFPS over in vivo protein expression include its open system, the elimination of reliance on living cells, and the ability to focus all system energy on production of the protein of interest. Over the last 60 years, the CFPS platform has grown and diversified greatly, and it continues to evolve today. Both new applications and new types of extracts based on a variety of organisms are current areas of development. However, new users interested in CFPS may find it challenging to implement a cell-free platform in their laboratory due to the technical and functional considerations involved in choosing and executing a platform that best suits their needs. Here we hope to reduce this barrier to implementing CFPS by clarifying the similarities and differences amongst cell-free platforms, highlighting the various applications that have been accomplished in each of them, and detailing the main methodological and instrumental requirement for their preparation. Additionally, this review will help to contextualize the landscape of work that has been done using CFPS and showcase the diversity of applications that it enables.
Keywords: cell-free metabolic engineering (CFME); cell-free protein expression (CFPE); cell-free protein synthesis (CFPS); cell-free synthetic biology; in vitro protein synthesis; in vitro transcription-translation (TX-TL).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

