The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro
- PMID: 31164685
- PMCID: PMC6547643
- DOI: 10.1038/s41598-019-44753-8
The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro
Abstract
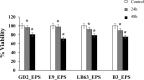
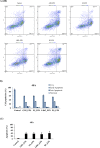
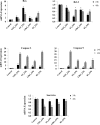
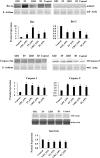
Colon cancer is one of the most common cancer around the world. Exopolysaccharides (EPSs) produced by lactobacilli as potential prebiotics have been found to have an anti-tumor effect. In this study, lyophilized EPSs of four Lactobacillus spp. for their impact on apoptosis in colon cancer cells (HT-29) was evaluated using flow cytometry. The relationship between capability of a lactobacilli-EPS to induce apoptosis and their monosaccharide composition, molecular weight (MW), and linkage type was investigated by HPLC, SEC, and NMR, respectively. Changes in apoptotic-markers were examined by qPCR and Western Blotting. EPSs were capable of inhibiting proliferation in a time-dependent manner and induced apoptosis via increasing the expression of Bax, Caspase 3 and 9 while decreasing Bcl-2 and Survivin. All EPSs contained mannose, glucose, and N-acetylglucosamine with different relative proportions. Some contained arabinose or fructose. MW ranged from 102-104Da with two or three fractions. EPS of L. delbrueckii ssp. bulgaricus B3 having the highest amount of mannose and the lowest amount of glucose, showed the highest apoptosis induction. In conclusion, lactobacilli-EPSs inhibit cell proliferation in HT-29 via apoptosis. Results suggest that a relationship exists between the ability of EPS to induce apoptosis and its mannose and glucose composition.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- The International Agency for Research on Cancer (IARC). Latest Global Cancer Data, 2018. World Health Organization (2018).
-
- Gueimonde M, Ouwehand A, Huhtinen H, Salminen E, Salminen S. Qualitative and quantitative analyses of the bifidobacterial microbiota in the colonic mucosa of patients with colorectal cancer, diverticulitis and inflammatory bowel disease. World J Gastroenterol. 2007;13:3985–3989. doi: 10.3748/wjg.v13.i29.3985. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

